Xanomeline–Trospium For Schizophrenia
Di: Ava
Background: The muscarinic receptor agonist xanomeline has antipsychotic properties and is devoid of dopamine receptor-blocking activity but causes cholinergic adverse events. Trospium is a peripherally restricted muscarinic receptor antagonist that reduces peripheral cholinergic effects of xanomeline. The efficacy and safety of combined xanomeline Formally known as KarXT, xanomeline-trospium chloride (Cobenfy) receives approval from the FDA for the treatment of schizophrenia in adults.
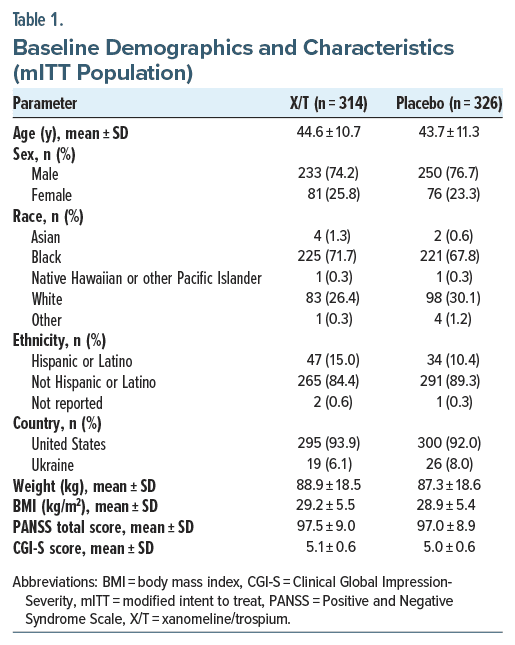
The US Food and Drug Administration (FDA) recently approved a new treatment for schizophrenia that relies on a novel mechanism of action. Oral xanomeline and trospium chloride, marketed as Cobenfy, is the first antipsychotic drug authorized to treat the disease via cholinergic receptors instead of PANSS subscale and Clinical Global Impression-Severity scores also improved significantly with xanomeline/trospium versus placebo. Subgroup analyses consistently favored xanomeline/trospium over placebo regardless of differences in participant age, sex, race, body mass index, and baseline PANSS total score.
Abstract Purpose: Describe xanomeline and trospium chloride efficacy and safety/tolerability for the treatment of schizophrenia using number needed to treat (NNT), number needed to harm (NNH), and likelihood to be helped or harmed (LHH).
Xanomeline/Trospium for Schizophrenia
Xanomeline-trospium is the first approved medication for schizophrenia in this new ther-apeutic class. This review describes the safety and effi-cacy of xanomeline-trospium in the treatment of adults with schizophrenia. Xanomeline and trospium were first developed in the 1990s and 1960s respectively, and in recent clinical trials they were tested as a fixed-dose combination given twice daily, which demonstrated safety and efficacy in treating schizophrenia.
Conclusion: Xanomeline/trospium showed improvement in agitation as measured by PANSS-EC in participants with acute exacerbations of schizophrenia. Xanomeline/trospium is the first in a new class of treatments for schizophrenia based on muscarinic receptor agonism.
A close look at xanomeline-trospium and CVL-231 (emraclidine) as potential targets for treating schizophrenia’s negative and cognitive symptoms with fewer medication side effects. OBJECTIVES: To expand prior network meta-analyses investigating the relative efficacy, safety, and tolerability of xanomeline and trospium, for the acute treatment of schizophrenia.
- Xanomeline-Trospium: A Novel Therapeutic for the Treatment of Schizophrenia
- TerXT: Combination of Xanomeline and Trospium Prodrugs for Schizophrenia
- Xanomeline/Trospium Chloride: First Approval
Xanomeline-trospium shows promising results for treatment of schizophrenia. Further studies with active comparators, larger sample sizes, and more patients with prominent negative symptoms are needed to corroborate efficacy and determine place in therapy. Author: Caryssa Drinkuth Editor: Keita Yokoyama Today, we will be covering Bristol-Myers Squibb’s Cobenfy (xanomeline and trospium chloride). On September 26, 2024, the Food and Drug Administration (FDA) approved Cobenfy for the treatment of schizophrenia in adults, designating Cobenfy as the first antipsychotic drug to utilize a novel mechanism of action for Cobenfy (xanomeline-trospium chloride) was approved for the treatment of adults with schizophrenia in 2024. Cobenfy is the first medicine approved for the
Summary Background New treatments with new mechanisms are urgently needed for people with schizophrenia. Xanomeline is a dual M1 and M4-preferring muscarinic receptor agonist that does not block D2 dopamine receptors, unlike all currently approved treatments for schizophrenia. Xanomeline–trospium (KarXT) combines xanomeline with the peripherally restricted muscarinic Efficacy of xanomeline and trospium chloride in schizophrenia: pooled results from three 5-week, randomized, double-blind, placebo-controlled, EMERGENT trials However, these medications often come with notable limitations and side effects. Recently, Cobenfy, a novel antipsychotic combining xanomeline and trospium chloride, received the United States (US) Food and Drug Administration (FDA) approval, marking a significant advancement in schizophrenia treatment.
FDA Approves Novel Schizophrenia Drug
The United States Food and Drug Administration approved xanomeline-trospium combination for schizophrenia on September-26-2024. We conducted a PRISMA 2020-compliant systematic review with random-effects meta-analysis on the efficacy and safety of xanomeline-trospium in randomized controlled trials i
Results The clinical development of bitopertin and pimavanserin was halted despite the early promises. Xanomeline-trospium chloride was recently approved by the FDA for the treatment of schizophrenia. Ulotaront showed mixed results, although analysis is ongoing. 1. Bristol Myers Squibb announces topline results from phase 3 ARISE trial evaluating Cobenfy (xanomeline and trospium chloride) as an adjunctive treatment to atypical antipsychotics in adults with schizophrenia. News release. April 22, 2025. The effect of xanomeline-trospium on acute symptom control in schizophrenia is straightforward. Across pivotal studies, xanomeline-trospium showed a moderate-size treatment effect against total symptoms (d=0.65), with somewhat lesser effects against negative symptoms (d=0.42) (3). The magnitude of symptomatic benefit from xanomeline-trospium is thus similar to
The muscarinic receptor agonist xanomeline improved cognition in phase 2 trials in Alzheimer’s disease and schizophrenia. We present data on the effect of KarXT (xanomeline–trospium) on Statistically significant and clinically meaningful results from clinical studies reveal that xanomeline and xanomeline-trospium are promising, innovative treatments for all dimensions of schizophrenia. Effectiveness of KarXT (xanomeline-trospium) for cognitive impairment in schizophrenia: post hoc analyses from a randomised, double-blind, placebo-controlled phase 2 study Colin Sauder 1 , Luke A
Psychopharmacology Why This Potential New Medication for Schizophrenia Is Important A new study reports results of a phase-3 clinical trial of xanomeline-trospium. Posted February 6, 2024
By combining xanomeline with the peripherally restricted muscarinic acetylcholine receptor antagonist trospium chloride, the xanomeline Xanomeline/trospium chloride (COBENFY™), formerly KarXT, is a first-in-class, oral, fixed-dose muscarinic agonist/antagonist combination being developed for use in schizophrenia and Alzheimer’s disease psychosis. Xanomeline is thought to confer efficacy by acting as an agonist at M1 and M4 muscarinic acetylcholine receptors in the brain, and
Schizophrenia is a severe mental illness that has its onset in late adolescence or early adulthood and is associated with significant dysfunction across multiple domains. The pathogenesis of schizophrenia remains unknown, but physiologic Xanomeline-trospium is currently under review for approval by the US Food and Drug Administration for the treatment of schizophrenia. Comprising xanomeline, a cholinergic agonist acting on acetylcholine muscarinic receptors M1 and M4, and trospium, added to mitigate peripheral cholinergic effects, this medication offers a potentially novel treatment mechanism
Xanomeline/Trospium Chloride: First Approval
Xanomeline/trospium chloride (Cobenfy) is the first medication for schizophrenia that the FDA approved in more than 50 years. Read more about its benefits, side effects and dosage.
What is trospium and xanomeline? Trospium and xanomeline is a combination medicine used in adults to treat schizophrenia. Trospium and xanomeline may also be used for purposes not listed in this medication guide. Background: New treatments with new mechanisms are urgently needed for people with schizophrenia. Xanomeline is a dual M 1 and M 4 -preferring muscarinic receptor agonist that does not block D 2 dopamine receptors, unlike all currently approved treatments for schizophrenia. Xanomeline-trospium (KarXT) combines xanomeline with the peripherally
Abstract Xanomeline/trospium chloride (COBENFY™), formerly KarXT, is a first-in-class, oral, fixed-dose muscarinic agonist/antagonist combination being developed for use in schizophrenia and Alzheimer’s disease psychosis. Xanomeline is thought to confer efficacy by acting as an agonist at M 1 and M 4 muscarinic acetylcholine receptors in the brain, and
- Xella Ytong-Porenschrauben _ Mineralische Baustoffe für Wohn- und Wirtschaftsbau
- X-Plane Endlich Mit Mehr Fps? Vulkan Vs. Opengl
- Wwe Mayhem 1.57.126 Apk Download
- Wärme- Und Stofftransport Bei Der Röstung Von Kaffeebohnen
- Xviii Airborne Corps Hhbn Sponsorship
- Wy7 Weinhandlung Und Bar , Die 10 besten Weinbars in Zürich
- Xdr Solution With Watchguard’S Threatsync
- Wwe Star Sara Lee’S Husband Reacts To Her Death
- Xbox Game Pass: Diese Spiele Könnt Ihr Im März Im Abo Spielen
- Xerostomia, Pôr Um Fim À Boca Seca
- X90J Series Tulajdonságok | SONY BRAVIA X90J REFERENCE MANUAL Pdf Download
- Xt.Com Kurs In Euro: Xt In Eur
- Xl 1200 Forty-Eight: Screaming Eagle Chisel Luftfilter Eintragen?
- Xiaomi Mi 6 Price In India 2024, Full Specs, Reviews, Offers