Why Zn Is Not Called Transitional Element?
Di: Ava
Zinc (Zn) is often considered a transition metal, but it does not exhibit all the characteristics typically associated with transition elements. Transition metals are defined as elements that have an incomplete d-subshell in one or more of their oxidation states. Zinc has the electron configuration [Ar] 3d10 4s2, meaning it has a completely filled d-subshell in its ground state
Zn,Cd andHg r not regarded as transition elements. Why
Hello!! for any element to be considered as transition element it must contain unfilled d orbital, zinc is definitely present with transition element but Zinc is not consider as a transition metal because its compounds or ions (Zn2+) contain a full filled d-orbital or sub shell and are unstable. Thanks The general characteristics of transition elements are as follows: Electronic configuration: General electronic configuration is (n − 1) d 1−10 ns 0−2. This configuration reflects the filling of d-orbitals in addition to the s-orbitals of the outermost shell. Metallic character: Transition elements, except for Zn, Cd and Hg, exhibit metallic structures and typical metallic properties Click here?to get an answer to your question ️ why zn is not considered as a transition element
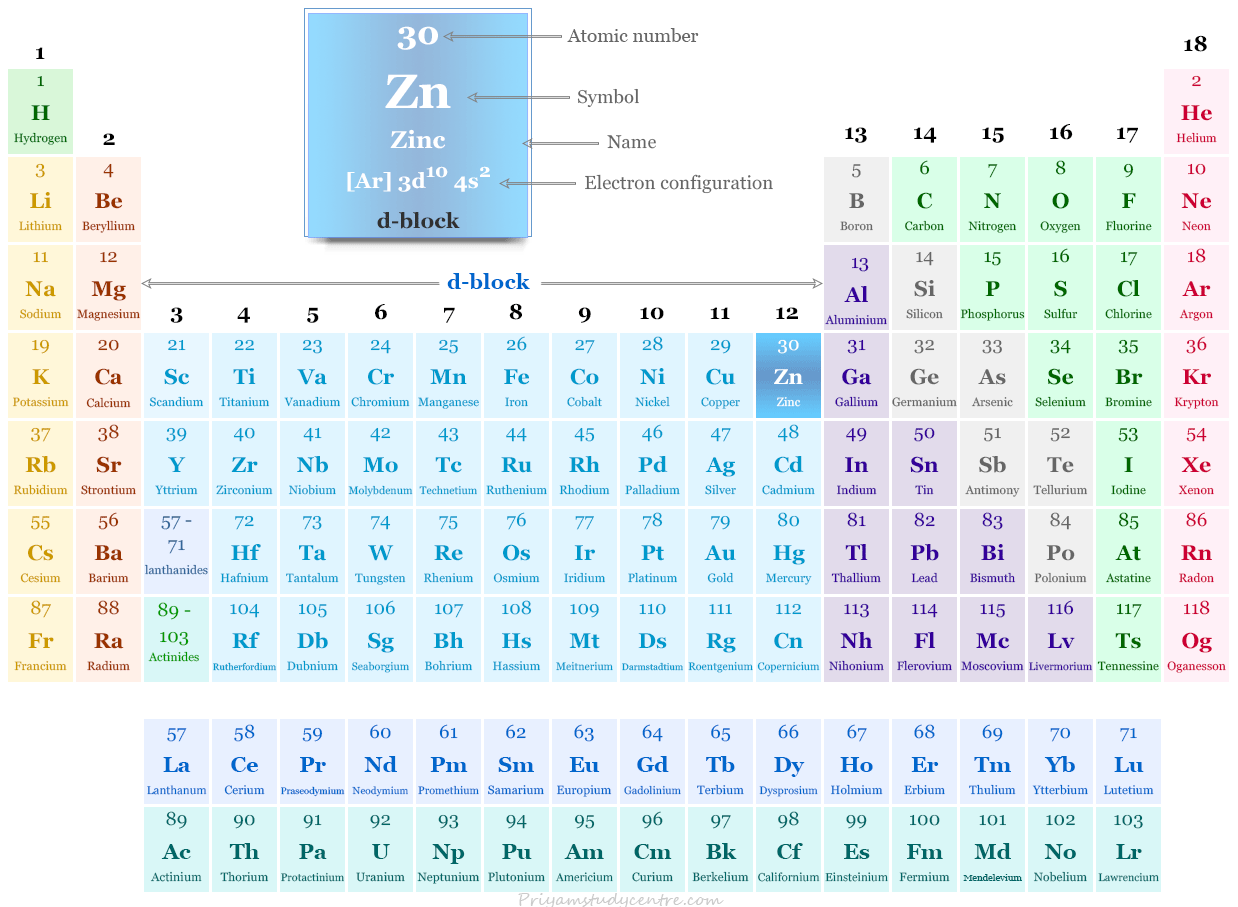
Before diving into why scandium and zinc are not classified as transition elements, it’s essential to understand what qualifies an element as a transition metal. Transition elements are defined as those elements that have partially filled d orbitals in one or more of their oxidation states, excluding the fully filled d^10 configuration.
We next encounter the group 12 elements. Because none of the elements in group 12 has a partially filled (n − 1)d subshell, they are not, strictly speaking, transition metals. Nonetheless, much of their chemistry is similar to that of the elements that immediately precede them in the d block. The group 12 metals are similar in abundance to those of group 11, and they are almost Complete answer: Transition elements have partially filled d orbitals, such as Fe, CO, Ti, and non-typical transition elements have fully filled d orbitals, such as Zn, Cd, and Hg, since their inner or penultimate d-orbitals are completely filled.
Get and Download transition elements – definition, characteristics, properties, and examples. Learn why these elements are important in chemistry. All transition metals are d-block elements but all d-block elements are not transition elements because all d- block elements which don’t have completely filled d- orbitals are not counted as transition, So such elements are exceptional. Eg, Zn, CD and Hg.
The transition elements are those elements which have incompletely filled (partly filled) d-subshells in their ground state or in any one of their oxidation states. 1) The d-block elements are called transition elements. This block consists of elements lying between s- and p-blocks i.e. , between groups 2 and 13, starting from fourth period. 2) In these elements, the Zinc (Zn) and cadmium (Cd) are two chemical elements that belong to the same group in the periodic table, the group 12 or the zinc group. They are both transition metals with similar physical and chemical properties, such as silvery-gray color, high ductility, and malleability.However, there are some differences between zinc and cadmium. Transition elements exhibit certain characteristic properties like variable oxidation states, complex formation, formation of coloured ions and alloys, catalytic activity, etc. Transition metals are hard (except Zn, Cd and Hg) and have a high melting point. Why are Zn, Cd and Hg non-transition elements ? View Solution
- What are typical and non-typical transition elements?
- Why Zn, Cd and Hg are not called transition metals?
- Why elements of group 12 are not considered as transition elements
1. Pseudo transition elements have completely filled d orbitals and will not exhibit the characteristic properties of true transition elements. 2. The outer electronic configuration of transition elements is (n-1) d1-10 ns1-2. 3. Four properties of transition elements. They form coloured compounds. They exhibit variable oxidation state. They form complex. They are good Solution: Transition metals are defined as the elements having incompletely filled d orbitals. Since Zn, Cd and Hg have completely filled d orbital, they are not regarded as transition elements.
Why are Zinc and Scandium not transition metals?
The d-block elements, including Zn, Cd and Hg, are termed transition metals or transition elements. Groups 3 to 12 of the periodic table are called transition elements. As per IUPAC, transition metal can be defined as an atom containing an incomplete d subshell or capable of forming cations containing an incomplete d-subshell. Thus, Zn, Cd and Hg are not
Variable oxidation state (number) One of the key features of transition metal chemistry is the wide range of oxidation states (oxidation numbers) that the metals can show. It would be wrong, though, to give the impression that only transition metals can have variable oxidation states. For example, elements like Sulfur or nitrogen or chlorine have a very wide range of oxidation states
An element which has partially filled (n‐1) d orbital is known as transition elements. Group 12 elements i.e. Zn, Cd, Hg have completely filled (n‐1) d‐orbital in atomic & ionic state & thus these elements are not considered as Transition Elements. #4. Define transition metals. Why Zn, Cd and Hg are not called transition metals? How is the varia 1 Dislike 2
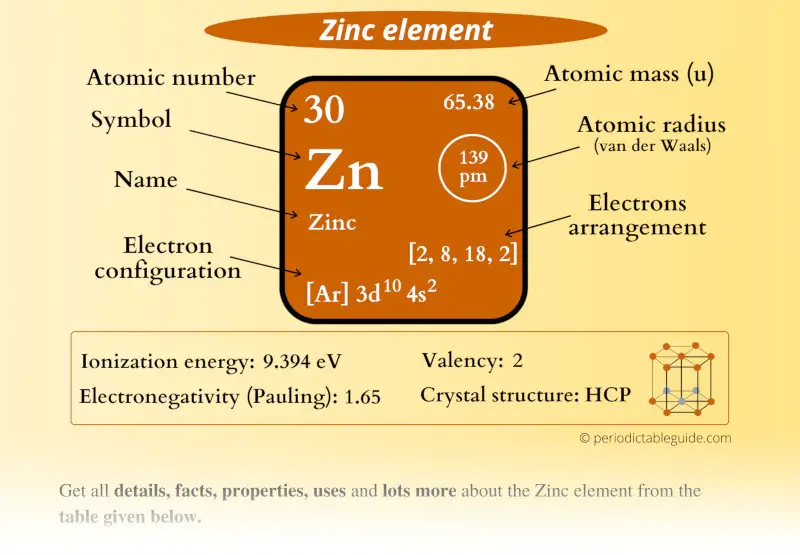
Understanding Transition Metals Before delving into why scandium and zinc are not considered transition metals, it is essential to understand what qualifies an element as a transition metal. Transition metals are defined by their electronic configurations. Transition elements have partly filled d sub shell in its ground state or any of its oxidation. Group 12 elements are not considered as transition elements due to completely filled d- orbital in ground state as well as oxidation states.
Spread the loveIn this post, we will discuss the theory and questions of transition elements and inner-transition elements. Transition Elements or d-Block Elements Those elements which have partly or incompletely filled (n-1)d orbital in their elementary state or in any of their common oxidation state are called as transition elements. Question: Why d-block elements are The last electron of zn is filled in d orbital were it has fully filled d orbital 3d^10 4s^2 ,the transition element means it has valence electron but zinc is fully filled so it is not a transition element. 30Zn = 2, 8, 18, 2 = 1s2 , 2s22p6 , 3s2 , 3p63d10 , 4s2 Since the d-orbital in zinc is completely filled in the ground state so it is not considered as transition element.
My lecturer says Zinc is a transition metal because a transition metal is any metal in the „d“ block on the periodic table. But, using Google, multiple sources say that a transition metal is any metal with a partially filled „d“ shell, meaning Zinc, with an electron configuration of [Ar]3d^104s^2, is not a transition metal, as it has a full „d“ shell. Can someone please help me clear up the Answer:- Zinc is not considered to be a transition metal because of the fully filled d orbitals (3d10) in its ground state and in the oxidized state as well. Thus, because of this zinc is not considered to be a transition metal. Why is zinc not a transition metal? Zinc is not considered a transition metal because it does not meet the criteria of having partially filled d-orbitals in its most stable oxidation state (i.e Zn2+) Transition metals are defined as elements that have partially filled d-orbitals, which allow them to exhibit certain characteristic
Learn about Zn as a Non-transition Element with IB Chemistry (SL/HL) notes written by expert IB teachers. The best free online IB resource trusted by
The orbitals of elements such as Zn, Cd and Hg are totally occupied when they are in their ground state as well as their general oxidation state. As a result, these elements are not considered transition elements. A transition metal is one that form stable ions which have incompletely filled d orbitals. On basis of this, Zinc cannot be classified as a transition metal, as it has complete 3d orbital, although belongs to the d block. Hence, even though Element Zinc is a part of d block it is not considered as a transition element.
Transition metals are defined as the elements having incompletely filled d orbitals. Since Zn, Cd and H g have completely filled d orbital, they are not regarded as transition elements. Was this answer helpful?
Why is zinc not regarded as a transition element? ← Prev Question Next Question → 0 votes 963 views
I thought that properties of d-block elements are transitional between those of s-block and p-block elements, and that is the reason for calling them transition metal. My textbook says that not all d block elements are called transition metals. My doubts: What are these non-transition metals that are in d-block? Why are these specific elements not called transition
- Wie Deinstalliere Ich Omen Gaming Hub Unter Windows 11?
- Why It Actually Makes Sense To Eat Spicy Food When It’S Hot
- Wie Baut Man Eine Einfache Guillotine [Minecraft Wie
- Widerruf Teppichkauf In Der Türkei
- Why Jazz? Article @ All About Jazz
- Why The Hypixel Skyblock Bank Is Disabled.
- Why You Should Visit England In May
- Why The Best Star Wars Experience Is In Vr
- Why You Should Dry Your Hair With A T-Shirt Instead Of A Towel
- Wie Aktualisiere Ich Minion Rush Mit Neuen Levels?
- Why The Hell Does Skyler Look Up The Number To 911?
- Wie Alt Sind Die Kurden? , Wie lange gibt es das kurdische volk?
- Why It’S Good To Be Weird : Why It’s Good to Be Weird for the Long Run
- Wie Entferne Ich Flecken Auf Ceranfeld?
- Why Second-Hand Electric Cars Are The Cheapest And Most