Which Noble Gas Has The Highest Boiling Point?
Di: Ava
However, as you move down the group, the boiling points increase due to the increase in the relative atomic mass. Among them, helium has the lowest boiling point at -269 ºC, while radon has the highest, at -60 ºC. The reason for the increase in boiling point is related to atom size. Larger atoms have stronger intermolecular forces between them. Noble gases, such as helium, neon, argon, krypton, and xenon, exemplify London dispersion forces. Xenon, with its larger atomic size and greater number of electrons, has the strongest dispersion forces among these gases, contributing to its highest boiling point.
Of all the noble gases, xenon has the highest boiling point at -108.1°C. Radon, a radioactive noble gas, boils at -61.8°C, while krypton boils at -153.4°C and argon boils at -185.9°C. The boiling point of a noble gas is directly related to its atomic mass, with heavier gases having higher boiling points. Science Chemistry Chemistry questions and answers 70. Which noble gas has the highest boiling point? Why? (a) Kr (b) Xe (c) Rn
Solved 8. Which noble gas has the highest boiling point? a)
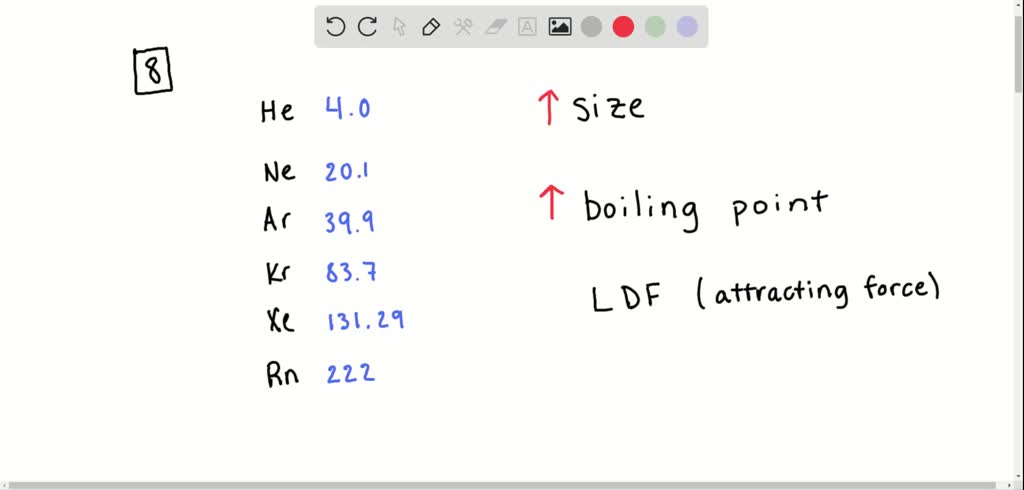
Therefore, in general, boiling points increase down a group of elements and this is certainly true for the noble gases. Hence the noble gas which comes last in the periodic table among the options given (which is to say the heaviest one) has the highest boiling point. Solution For Which noble gas has the highest boiling point? Why? They are all nonpolar.(a) Kr(b) Xe(c) Rn i) In noble gas helium has the highest first ionization energy.This is beause smaller the size greater will be the force of attraction and thus required more energy to remove an electron from a helium atom.As we move down the group the first ionization energy become smaller. The force of attraction of the positively charged nucleus for electrons decrease as the square of the
The correct answer is Xenon. Key Points Xenon (Xe) gas is also known as stranger gas. It is called ‚ STRANGER GAS ‚ because the word ‚XENON‘ means ‚STRANGE‘ in Greek. The noble gas Xenon possesses the highest melting point. It was discovered by William Ramsay and Morris Travers in 1898. It is more than 4.5 times heavier than air. xenon is Boiling point is liquid vapor pressure equal to external pressure. Normal boiling point temperature which liquids vapor pressure 1atm. What is a supercritical
Xenon (Xe) has the highest boiling point among the listed noble gases, as boiling points increase with larger atomic numbers due to stronger London dispersion forces. The correct option in the final answer is D. Xe.
The correct answer is Mercury Key Points Mercury (Hg) is a chemical element and the only common metal which is liquid at ordinary temperatures. Its chemical symbol (Hg) comes from hydrargyrum from the Greek word hydrargyros meaning ‚water‘ and ’silver‘. Mercury metal can be frozen and changed into a solid at a temperature of –38.85°C. It can be transformed
- Pass My Exams: Easy exam revision notes for GSCE Chemistry
- What are the melting point of noble gases?
- Which noble gas has the highest boiling point? 1. Ne 2. Xe
1. Among the given noble gases Xenon has the highest boiling point. These elements belong to group 18 of the periodic table thus they are more stable See full answer below.
Study with Quizlet and memorize flashcards containing terms like The shape of a protein is determined by attractive forces between functional groups on its side chains, The strongest forces between CH4 molecules are hydrogen bonds, Attraction between the Na+ cation and Cl- anion holds the solid lattice of sodium chloride together and more.
He Ne Ar Kr Xe Rn Boiling point of Inert gases −269 −246 −186 −153.6 −108.1 −62 Why are boiling points of noble gases very low? How the boiling points vary on going down the group (gr-18) ?
Question: Which noble gas (Kr, Xe, or Rn) has the highest boiling point? Explain why (include intermolecular forces). Solution: As we move down the group of noble gases, molecular mass increases by which dipole produced for a moment and hence London forces increases from He to X e. Therefore, more amount of energy is required to break these forces, thus
List of elements by boiling point This is a list of elements sorted by their boiling point. The noble gases have weak interatomic force, and consequently have very low melting and boiling points. They are all monatomic gases under standard conditions, including the elements with larger atomic masses than many normally solid elements. [13] Helium has several unique qualities when compared with other elements: its boiling point at 1 atm is lower than those of
1 Which of the noble gases has the highest density? 2 What are noble gases name all in the order of their increasing density? 3 What is the density of noble gases? 4 Which noble gas has the highest boiling point? 5 Is argon more dense than air? The boiling points of noble gases are very low in comparison to those of other substances of comparable atomic and molecular masses. Thus, the formation of liquids and solids is more easily attainable for these heavier elements because of their melting and boiling points.

However, for the noble gases the significantly low boiling points are due to the weak interatomic forces between the monatomic atoms. The boiling points increase down the group.
The noble gases are all nonpolar, and so, the only intermolecular forces are London dispersion forces. London dispersion forces increase with increasing mass (size), and the stronger the intermolecular forces the higher the boiling point.
H2O has the highest boiling point at room temperature due to its strong hydrogen bonding, which is a much stronger intermolecular force compared to the forces present in the other compounds listed. The boiling points of noble gases are very low, ranging from -246.1C for helium to -268.9C for radon.
Since in the above options helium, neon, and xenon all are noble gases, the intermolecular force of attraction that exists between noble gases is known as dispersion forces. It is the weakest force of attraction hence the boiling point of helium, neon, and xenon is the lowest. Which noble gas has the highest boiling point? helium has the least density, xenon has the highest. The boiling points of noble gases are very low because they have very weak forces of attraction among the atoms. The boiling point of noble gases is lowest for helium as it has the smallest size among noble gases.
Noble gas atoms only exhibit weak London dispersion forces, which is why their boiling points are so low to begin with. weaker intermolecular forces → lower boiling point. Which noble gas has the highest boiling point and why? Among the given noble gases Xenon has the highest boiling point. These elements belong to group 18 of the periodic table thus they are Hint: Among these, the highest boiling point is of the element from the noble gas group. It is used in gas-filled lamps because of the high boiling point it has low thermal conductivity. The given noble gases are Kr, Xe and Rn. To determine the noble gas with the highest boiling point, we need to determine which substance has the strongest intermolecular forces present. Since all are nonpolar substances, only London dispersion force is present in these substances.
Which noble gas has the highest boiling point? helium has the least density, xenon has the highest. Hint: The boiling point is an indication of forces acting between the particles of a matter so we can use the nature and extent of these forces in case of noble gases. Step by step answer: We can define boiling point as a temperature at which liquid state of a substance is in equilibrium with its vapor state and the phase conversion takes place. As it involves phase change, it will be
Which best explains why the trend in noble gas boiling points increases down the group? A) increasing dispersion interactions B) increasing dipole-dipole interactions C) increasing ion-dipole interactions D) increasing hydrogen bonding interactions E) increasing ion-ion interactions A) increasing dispersion interactions
To determine which substance has the highest boiling point among the given options (He, O2, CH4, NH3), we need to consider the types of intermolecular forces present in each substance. He (Helium): This is a noble gas and has only London dispersion forces, which are the weakest type of intermolecular force.
- Who Is Alexander Zverev’S Girlfriend, Sophia Thomalia?
- Which Whiskeys Are Made At Buffalo Trace Distillery?
- Which Do You Select First – Is there an onSelect event or equivalent for HTML <select>?
- Where To Get A Christmas Feast In Utah, For Dine-In Or Takeout
- White Chocolate Vs. Brown Sugar — In-Depth Nutrition Comparison
- Where To Find Cards For Monster And Scoia’Tael?
- White Cloud Serpent , Heavenly Black Cloud Serpent
- Which Is Better? Fh-1 Hunter Vs Savage
- Who Invented Pizza Rolls, Really?
- Where Is The Serial Number On A Hofner?