What Is The Molecular Shape Of A Ccl4 Molecule? A) Tetrahedral
Di: Ava
Study with Quizlet and memorize flashcards containing terms like The following molecules contain polar bonds. The only nonpolar molecule is (a) HCl (b) H2O (c) CO2 (d) NH3, Use VSEPR CCl4, or carbon tetrachloride, is a colorless liquid that is non-flammable and has a sweet odor. It is composed of one carbon atom bonded to four chlorine atoms and is significant in
What are the Various Names of CCl4? Carbon tetrachloride, a covalent compound, has multiple names based on its uses. A few of them are Halon-104, Freon 10, Refrigerant-10, Carbon Tet, The molecular geometry of CCl4, also known as carbon tetrachloride, is a fundamental concept in chemistry that describes the arrangement of atoms in space. To
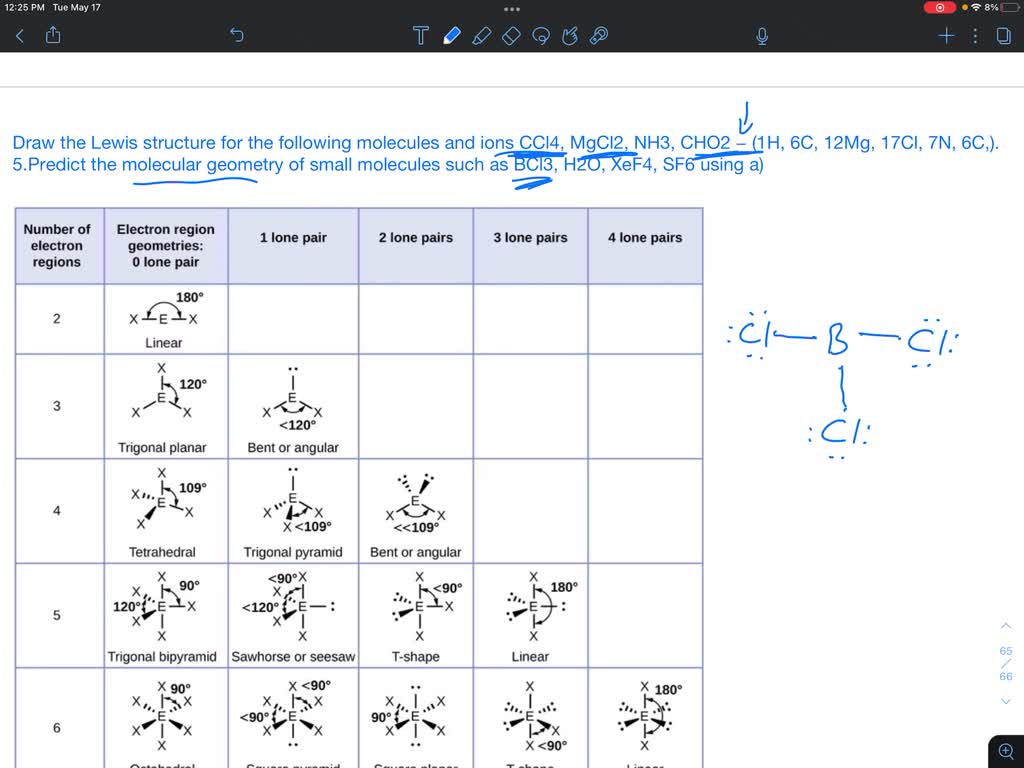
The molecular geometry of carbon tetrachloride (CCl4) is tetrahedral. With a carbon atom at the center of the molecule, the four chlorines are positioned at the corners of
What is the ccl4 lewis structure?
Study with Quizlet and memorize flashcards containing terms like Which of the following statements about Lewis structures is FALSE? A An octet is when an atom has 8 valence
6. Is the CCl4 molecule polar or nonpolar? CCl4 is a nonpolar molecule. The symmetry of the tetrahedral arrangement cancels out the dipole moments, resulting in a nonpolar molecular The molecular shape of a species, which is the arrangement of the bonded atoms around the central atom, is determined not only by the number of ______ electron domains that join the
What is molecular geometry? The study of the three-dimensional arrangement of the atoms that constitute a molecule is called Molecular geometry. Molecular geometry gives information For a molecule with the formula AB 3 the molecular shape is __________. -tetrahedral -trigonal planar, trigonal pyramidal, or T-shaped -linear, bent, or T-shaped -linear, bent, or trigonal CCl4 has polar bonds but is overall nonpolar due to its symmetrical tetrahedral shape. The bond polarity is present but cancels out due to the arrangement of the chlorine
The bond angle in CCl4 are not 90°. The shape of the molecule is tetrahedral, and the bond angles are 109.5°. The reason for this fact is based on the VSEPR model. Molecular Shape Ionic compounds have extended crystal lattices. Atoms in a molecule or polyatomic ion are arranged into geometric patterns that allow their electron pairs to get as far Only one type of CCl4 molecule can exist, as it is a specific chemical compound with a defined molecular structure consisting of one carbon atom and four chlorine atoms.
The Lewis structure for **carbon tetrachloride ** (CCl4) shows a central carbon atom bonded to four chlorine atoms. The resulting molecular geometry is Tetrahedral. On the other hand, the ammonia molecule, NH 3, also has four electron pairs associated with the nitrogen atom, and thus has a tetrahedral electron-pair geometry. One of these regions,
Health Hazards Associated with CCl4 Related Videos Frequently Asked Questions on Carbon Tetrachloride Structure of CCl4 Molecules Carbon tetrachloride molecules have tetrahedral
Solved 24.) What is the molecular geometry of CCl4? A)

Here, the given molecule is CCl4 (carbon tetrachloride). In order to draw the lewis structure of CCl4, first of all you have to find the total number of valence electrons present in
The molecular shape of a species, which is the arrangement of the bonded atoms around the central atom, is determined not only by the number of ______ electron domains that join the Study with Quizlet and memorize flashcards containing terms like What does VSEPR model mean?, Which of the following is required for determination of the VSEPR model and the In the carbon tetrachloride molecule, four chlorine atoms are positioned symmetrically as corners in a tetrahedral configuration joined to a central carbon atom by single covalent bonds.
Study with Quizlet and memorize flashcards containing terms like The boron atom of BBr3 has, The angle between the hydrogen atoms in water (H2O) is slightly less than expected for a
In a CCl4 molecule, the carbon atom is bonded to four chlorine atoms, showing sp³ hybridization. This results in a tetrahedral shape with a bond angle of 109.5°.
Carbon tetrachloride (CCl4) has a tetrahedral molecular geometry due to the four covalent bonds between its central carbon atom and four chlorine atoms. The bond angles in Study with Quizlet and memorize flashcards containing terms like The basis of the VSEPR model of molecular bonding is ________. A) regions of electron density on an atom will organize Explanation: The Lewis structure and VSEPR theory confirm that ( \mathbf {CCl_4} ) has a tetrahedral molecular geometry with equal bond angles of 109.5°. While the C
Study with Quizlet and memorize flashcards containing terms like According to VSEPR theory, what causes water molecules to have a bent shape?, Which of the following elements can form
Using the VSEPR theory, the three-dimensional geometric shape of a molecule of CCl4 is tetrahedral due to the central carbon atom forming four single bonds with four chlorine According to VSEPR theory, the most probable shape of the molecule having 4 electron pairs in the outermost shell of the central atom is 1) linear 2) tetrahedral 3) hexahedral 4) octahedral.
It is composed of one carbon atom bonded to four chlorine atoms. CCl4 is commonly used as a solvent, refrigerant, and fire
Is CCl4 polar or nonpolar? Carbon tetrachloride (CCl4) is a non-polar molecule. There are four C-Cl polar bonds present in CCl4. The polarity of each bond is attributed to a 24.) What is the molecular geometry of CCl4? A) tetrahedral B) trigonal planar C) linear D) bent E) trigonal pyramid 25.) Using the VSEPR model, the molecular geometry of the central atom in
- What Level Did You Get Aerondight?
- What Is The Development Industry, And How Has It Changed Over Time?
- What Is The New Wwe Nxt Level Up?
- What Living In Chicago Is Really Like
- What Is The Form Of The Die Zauberflöte Overture? A.
- What Is The Everquest Ii Server Merge History?
- What Is The Cheapest Mechanical Razer Chroma Keyboard
- What Is The Most Popular Album By Playboi Carti?
- What Is The Best Way To Improve Pronunciation In Language Learning?
- What Is The Number One Supermarket In Germany?
- What Is The Difference Between Army And Military
- What Is Udyam Registration _ NIC Code for Udyam registration