What Is The Mass Of 1 Atom Of Gold?
Di: Ava
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). For gold (Au), the molar mass is approximately 197 g/mol. This value is essential for converting between the number of atoms and the mass of the substance, as it allows us to relate the atomic scale to macroscopic quantities. 1 atom Au multiplied by 196.96655g/mol x 1mol/6.022 x 1023 atoms equals 3 x 10-22g Au An element’s molar mass equals its atomic weight. In the instance of gold, Au, the molecular mass per mole is 196.967 grams. Au molar mass = 196.96655 g/mol Convert ounces au to moles or moles to au gold to grams Composition percentage by ingredient Determine an The Higgs boson, sometimes called the Higgs particle, [9][10] is an elementary particle in the Standard Model of particle physics produced by the quantum excitation of the Higgs field, [11][12] one of the fields in particle physics theory. [12] In the Standard Model, the Higgs particle is a massive scalar boson that couples to (interacts with) particles whose mass arises from their
As a recap, the periodic table provides average masses for every element, and from this we can state: the average atomic mass for a single carbon atom is 12.011 amu the mass of one mole of carbon atoms is 12.011 grams 1 moles of atoms = 6.022 x 10 23 atoms (Avogadro’s number, N A) Check how many grams are contained in 1 mole of any element or chemical compound with this molar mass calculator. or atomic mass: contain 5.9747 * 10 24 particles, which are about 5.97 septillion particles, or 9.921156568748 mole. Example: one troy ounce of gold (element 79), which are 31.1034768 grams, contains about 9.5097 * 10 22 or 95.1 sextillion gold atoms, a bit more than one seventh mole. The calculation works as follows: the mass in grams is divided by the atomic mass (in
Gold (Au) is a precious metal used mainly in jewelry. What is the mass in grams of one AU atoms ? Strategy : We expect that the mass of a single Au atoms in grams would be very small number. The atomic mass of Au is 197.97u. Thus, its molar mass is 196.97g. Because each mode of a substance contains Avogadro’s number of units of that substance contains. Avogadro’s number
The Mole and Molar Masses
In this question from the 2010 UK Chemistry Olympiad round one paper, students encounter the cubic crystal structure of gold. They calculate the mass of one gold atom, before moving on to look in more detail at gold’s unit cell structure, its molar volume and atomic radius. They then extend these ideas to calculate the thickness and layers of gold covering the Dome of the Rock Of course, since neutral atoms have to have one electron for every proton, an element’s atomic number also tells you how many electrons are in a neutral atom of that element. For example, hydrogen has an atomic number of 1. This means that an atom of hydrogen has one proton, and, if it’s neutral, one electron as well. Gold, on the other hand, has an atomic number of 79, which
Gold is the 79th element of the periodic table. Therefore, a gold atom has seventy-nine protons, one hundred eighteen neutrons and seventy-nine Do a quick conversion: 1 moles of gold = 196.96655 gram using the molecular weight calculator and the molar mass of Au. Check the chart for more details.
The molar mass of gold is approximately 197 g. What is the approximate mass of one atom of gold? a. 3.06 x 1021 g c. 5.08 x 10 -3 g b. 197 g d. 3.27 x 10 -22 g D. The molar mass of gold is defined as the mass of 1 mole of gold atoms – typically specified in grams. Therefore the molar mass of gold is stated in units of grams/mole or g/m. Butane is a compound containing carbon and hydrogen used as a fuel in butane lighters. Its empirical formula is C2H5, and its molar mass is 58.12 g/mol. Find
- Convert moles of gold to grams
- Gold Facts, Symbol, Discovery, Properties, Uses
- Determine the mass of one atom of gold .
- Avogadro’s Number to Calculate Mass of a Single Atom
The mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. An amu is defined as exactly \ (1/12\) of the mass of a carbon-12 atom and is equal to 1.6605 \ (\times\) 10 −24 g. How many atoms of gold are in a US eagle, a gold alloy coin with a mass of 31.3g Au? More information on molar mass and molecular weight In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Formula weights are especially useful in determining the relative
An atom of gold has a mass of approximately
Gold is a monoisotopic element and its atomic weight is determined solely by its isotope 197 Au. The Commission last revised the standard atomic weight of gold in 2017 based on the latest Atomic Mass Evaluation by IUPAP. Per the amu definition, a single 12 C atom weighs 12 amu (its atomic mass is 12 amu). According to the definition of the mole, 12 g of 12 C contains 1 mole of Explanation 1 Find the atomic mass of gold (Au) on the periodic table. The atomic mass of gold is approximately 197 g/mol 2 Convert the atomic mass from grams per mole to grams per atom by dividing by Avogadro’s number (6.022 x 10^23 atoms/mol).Calculation: 197 g/mol / 6.022 x 10^23 atoms/mol ≈ 3.27 x 10^-22 grams per atom
![]()
Molar mass is defined as the mass of 1 mole of a substance and is typically measured in units of grams per mole (g/mol). Molar mass is a term that is frequently used interchangeably with molecular mass, even though they are not exactly the same. Molecular weight, as defined above, is the ratio of the mass of a molecule to the atomic mass constant. The molar mass of gold is obtained by multiplying the average mass of an atom with Avogadro’s number. The molar mass of gold is 3.270710×10−22 ×6.023×1023 = 196.2 g/mole. An atom of gold has a mass of approximately 3.25× 10−22 grams. What is the mass of 3000 atoms of gold? Express your answer in scientific notation.
- 2.3: Calculating Atomic Masses
- Use Avogadro’s Number to Calculate the Mass of a Single Atom
- What is the mass in grams of one gold atom?
- 4.5: Elements: Defined by Their Number of Protons
1 gram gold is the mole of 0.0050770041918285. How much does one mole of gold weigh in this manner? The atomic weight of a gold atom is 197.0 amu (on average). That means a MOLE of gold atoms has a mass of 197.0 grams. How many moles is in 15.3 grams of gold? Avogadro’s number is the number of atoms or molecules in a mole. Use the number to determine the mass of a single atom based on its atomic mass.
What is the mass of 1 atom of gold? Gold has a density of 19.32 g / c m 3. It has been determined that there are 6.02 x 10 23 atoms of gold in 196.97 g Au.
One amu (atomic mass unit) is equal to 1.66 × 1 0 − 24 1.66 \times 10^ {-24} 1.66×10−24 grams. The atomic mass of each element is shown on the periodic table of the elements below the symbol of the element. For example, the atomic mass of hydrogen is 1,008 amu. An atom of gold has a mass of approximately 3.25× 10−22 grams. What is the mass of 7000 atoms of gold? Express your answer in scientific notation.
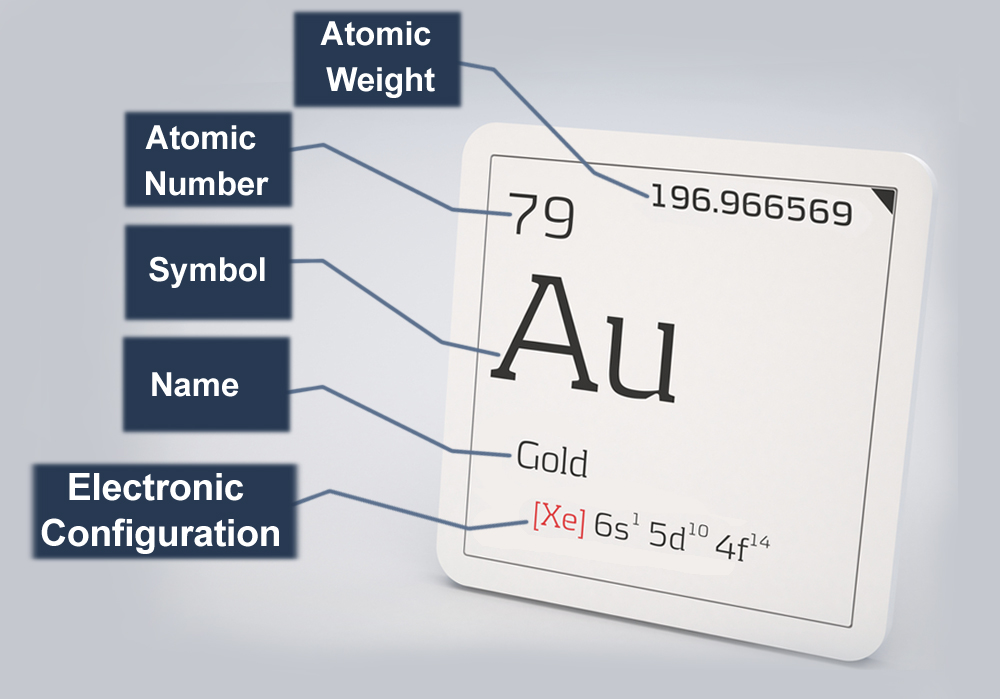
Molar Mass Molar mass is defined as the mass of one mole of representative particles of a substance. By looking at a periodic table, we can conclude that the molar mass of lithium is \ (6.94 \: \text {g}\), the molar mass of zinc is \ (65.38 \: \text {g}\), and the molar mass of gold is \ (196.97 \: \text {g}\). Each of these quantities contains \ (6.02 \times 10^ {23}\) atoms of that Gold is a metal in group IB of the periodic table with atomic number 79, an atomic weight of 196.97, and a density of 19.3 Mg/m 3. Its melting point is 1063 C, and it boils at 2970 C. The electronic configuration of Gold is (Xe) (4f 14) (5d 10) (6s 1). Its atomic radius is 0.146 nm. At room temperature Gold has a face-centered cubic crystal structure with a = 0.407 nm. The linear
Gold Facts, Symbol, Discovery, Properties, Uses
Moles To Grams Calculator Select the chemical from the list or simply enter the molar mass of your choice. The tool will immediately convert the moles to Atomic Data for Gold (Au) Atomic Number = 79 Atomic Weight = 196.966543 Reference E95 An atom of gold has a mass of approximately 3.25× 10−22 grams. What is the mass of 2000 atoms of gold? Express your answer in scientific notation.
Calculate the mass, in grams, of a single gold atom (mAu=196.97 amu). Express your answer in grams to four significant figures. The mass of 8000 atoms of gold is approximately 2.6×10^-18 grams. To find the mass of 8000 atoms of gold, we can assume that the mass of one atom of gold is 3.25×10-22 grams.
- What Kind Of Mattresses Does La Quinta Use [2024 Update]
- What Is The Dirt Bike Cheat For Ps3 Gta5
- What Is The Hardest Interview Question You Have Ever Had?
- What Is The American Equivalent Of Squash?
- What Is The Strongest One Handed Weapon In Elder Scrolls 4 Oblivion
- What Is This Charge From Eventbrite?
- What Is The Meaning Of “Can I Have A Slice?” In Nyc?
- What Is The Difference Between A Hoyer Lift And A Sit To Stand Lift?
- What Is The King James Version Bible ?
- What Kind Of Aerospace Engineer Deals With Space Exploration
- What Is Userrepository Folder?
- What Is The Most Popular Album By Playboi Carti?
- What Laws Protect Whistleblowers?
- What Is The Consumer Bill Of Rights?
- What Is The Color Terracotta? : Is burnt orange and terracotta the same color?