What Does The Subscript Of Atomic Orbital Mean?
Di: Ava
Two new components: parity and reflection Molecular orbitals are more complex than atomic ones and require more modifiers to completely define. Parity (sometimes called “inversion”) tells you if the orbital is symmetric or anti‐symmetric when an inversion operation is performed. The symmetry notation u and g are sometimes used when describing molecular orbitals. This refers The shape and size of an orbital can be determined from the square of the wave function Ψ2. Atomic orbitals have distinctive shapes; all are centered on the atomic nucleus. The most commonly encountered orbitals in elementary quantum chemistry are the orbitals corresponding to the s, p, and d subshells: these orbitals are named the s, p, and d orbitals. In the ground
What does the subscript mean in electron configuration? Each occupied sublevel designation is written followed by a superscript that is the number of electrons in that sublevel. For example, the hydrogen configuration is 1s1, while the helium configuration is 1s2. What does the letter N represent in electron configuration?
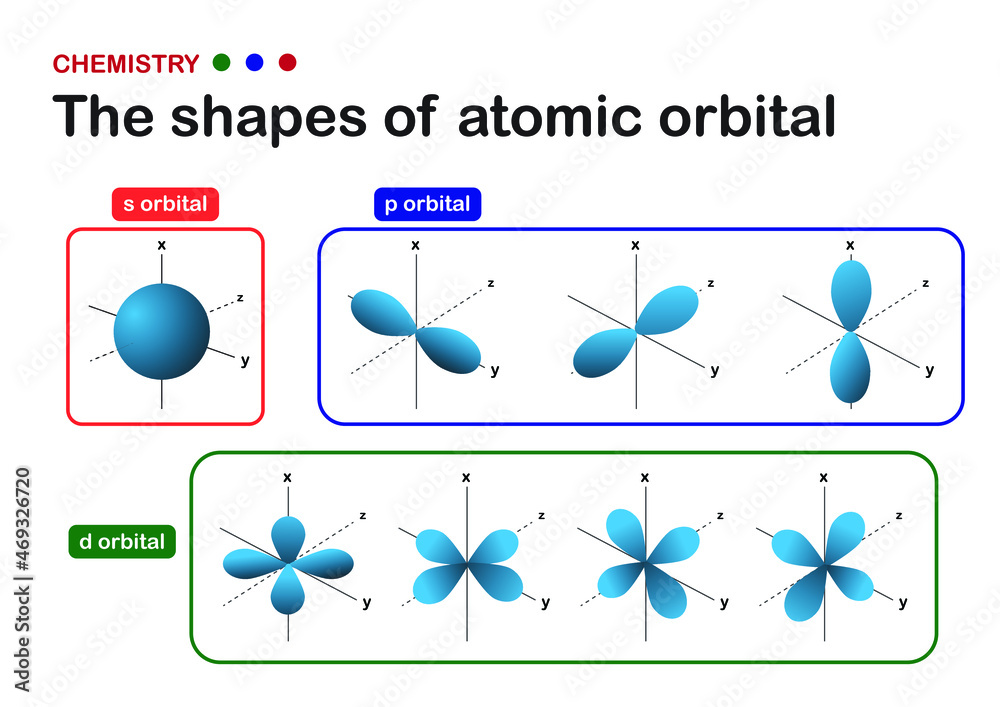
Why are do some electron orbitals have a negative number in the subscript (i.e. f-1), some a positive (f1), and some have a 0 (f0)? What do these numbers tell you about the atom? I know that these orbitals have different shapes, but I was wondering if they had any other effect on the atom/ion and if there was a reason that they were put in the order that they are in now. A molecular orbital that forms when atomic orbitals or orbital lobes with the same sign interact to give increased electron probability between the nuclei due to constructive reinforcement of the wave functions. In contrast, electrons in the \ ( \sigma _ {1s}^ {\star } \) orbital are generally found in the space outside the internuclear region.
Chapter 2.5: Atomic Orbitals and Their Energies
Figure 2.5.11 Orbital Penetration A comparison of the radial probability distribution of the 2s and 2p orbitals for various states of the hydrogen atom shows that the 2s orbital penetrates inside the 1s orbital more than the 2p orbital does.
The electrons are arranged in an elaborate system of atomic orbitals and the position of each electron is controlled by a set of four quantum numbers. Each of these numbers has a special meaning, such as the shell (principal energy level) at which the electron’s orbital is located, as well as the subshell, which is the type of orbital present
First, notice that there are the same number of molecular orbitals as there are atomic orbitals. Second, notice that each orbital in the diagram is rigorously
An atomic orbital is a mathematical function, known as a wave function, that describes the three-dimensional region around an atom’s nucleus where there is the highest probability of finding an electron. Unlike the fixed paths suggested by older models, an atomic orbital represents a probability cloud whose shape and energy are defined by a specific set of quantum numbers. Review Does the ground state beryllium atom contain any unpaired electrons? Why does one 2s electron in Be get promoted to a 2p orbital? What is the geometry of the two sp orbitals?
- What does the following symbol stand for?$ {H_2}
- Antibonding Molecular Orbital
- What does a subscript in chemistry tell you?
Like an atomic orbital, a molecular orbital can hold a maximum of two electrons with opposite spins. The shapes of molecular orbitals obtained by merging two atomic orbitals resemble the shapes of the atomic orbitals. When the orbitals merge to give a molecular orbital, they overlap in a region of space common to the bonded nuclei. Hybridized orbitals and unhybridized orbitals are two different types of atomic orbitals that play a crucial role in understanding the bonding and molecular geometry of molecules. Hybridized orbitals are formed by the mixing of atomic orbitals of similar energy levels, resulting in new orbitals with different shapes and orientations.
How do you read numbers in chemistry? Each element is represented by its atomic symbol in the Periodic Table – e.g. H for hydrogen, Ca for calcium. If more than one atom of a particular element is present, then it’s indicated by a number in subscript after the atomic symbol — for example, H2O means there are 2 atoms of hydrogen and one of oxygen.
4.2.1: Molecular Orbitals
Each orbital can hold a maximum of two electrons if these electrons have antiparallel spin. With each orbital we can associate an orbital energy εi. According to the aufbau principle, the state of lowest total energy is reached if the orbitals are filled in the order of increasing orbital energy. What does subscripts and superscripts mean in chemistry? The atomic number is written as a subscript on the left of the element symbol, the mass number is written as a superscript on the left of the element symbol, and the ionic charge, if any, appears as a superscript on the right side of the element symbol.
Each orbital is oriented along the axis indicated by the subscript and a nodal plane that is perpendicular to that axis bisects each 2p orbital. The If there was a subscript s after the H2O, it would mean that H2O is in a solid form as ice. If there was a subscript l it means that H2O is in the liquid form as water.
Atomic Orbital Essentials Atomic orbital are regions of space where the electrons are located. There are numerous possible orbitals within an atom, and each can accommodate up to two electrons. There are several types of orbital. These differ in their shape. And they differ in which shells they may be found in. Each orbital is oriented along the axis indicated by the subscript and a nodal plane that is perpendicular to that axis bisects each 2 p orbital. The phase of the wave function is positive (orange) in the region of space where x, y, or z is positive and negative (blue) where x, y, or z Short answer: You should move everything through the centre of symmetry. That’s why it’s a centre of symmetry. On the other hand, note that classifying orbitals just by g and u does not make much sense. You should always consider the entire molecule’s point group and thus always consider which (set of) irreducible representation (s) an orbital or a set of orbitals
4 d atomic orbitals There are five 4 d orbitals. These are labelled 4d xy, 4d xz, 4d yz, 4 dx2-y2 and 4 dz2. The 4 dz2 name is an abbreviation for 3 d(3z2–r2). Four of these functions have the same shape but are aligned differently in space. The fifth function (4 dz2) has a different shape. Molecular orbital theory is a conceptual extension of the orbital model, which was so successfully applied to atomic structure. As was once playfully remarked, Figure 7.3 The different types of atomic orbitals, with labels. The order of the orbitals in terms of energy is valid for He to Ar (discussed at the end of this article). There is a bit of a historical background regarding the way in which atomic orbitals are labelled (what s, p and d mean) but knowing the details will not really further our insight at this stage. Reading the orbital
What does "subscript" mean?
What does the following symbol stand for?$ {H_2} $ . Ans: Hint :Recall that the symbol $ H $ stands for hydrogen in the periodic table. Indicating 2 as subscript will mean that the atoms of hydrogen are combining to form a diatomic molecule. Every Sometimes the fundamental physical constants are used as though they were units, but they are still given italic symbols. However the electronvolt, eV, and the unified atomic mass unit, u, have been recognized as units by the Consultative Committee on Units of the BIPM and they are accordingly given roman symbols.
What does subscript mean in science? Subscripts are numbers that come after a symbol and below. Subscripts tell you the number of. atoms of that element. If an element does not have a subscript, then it is understood that the subscript is 1.
What does n L value mean? According to (n+l) rule: Orbital which has the least value of (n+l) will be filled first to the electrons. Example: 3s
An atomic orbital, which is distinct from an orbit, is a general region in an atom within which an electron is most probable to reside. The quantum mechanical model specifies the probability of finding an electron in the three-dimensional space around the nucleus and is based on solutions of the Schrödinger equation.
Study with Quizlet and memorize flashcards containing terms like Look at the aufbau diagram, figure 5.5. Which atomic orbital is of higher energy, a 4f or a 5p orbital?, In an electron configuration, what does a subscript stand for?, In an electron configuration what does the sum of the subscripts equal? and more. This page explains electron configurations as a simplified notation for representing electron arrangements in atoms, using superscripts to denote Typography of atomic orbital subscripts p_x Ask Question Asked 8 years, 6 months ago Modified 8 years, 6 months ago
Thus every molecular orbital for a homonuclear molecule must be either symmetric or antisymmetric with respect to the inversion operator. Orbitals which are left unchanged by the operation of inversion (are symmetric) are labelled with a subscript g, while those which undergo a change in sign (are antisymmetric) are labelled u.
Discover the antibonding orbital of the molecular orbital theory. Read a summary of the theory and its methods, and understand its role in
There are s, p, d, and f atomic orbitals each associated with an energy level and corresponding quantum numbers. Learn about periodic table nomenclature, element notation, and the use of subscripts, coefficients, and superscripts. In molecular physics, the molecular term symbol is a shorthand expression of the group representation and angular momenta that characterize the state of a molecule, i.e. its electronic quantum state which is an eigenstate of the electronic molecular Hamiltonian. It is the equivalent of the term symbol for the atomic case. However, the following presentation is restricted to the
What does the superscript 2+ mean in ca2+? If a charge is present, it’s indicated in superscript, with a sign (+/-) and a number if more than one charge is present. For example, calcium ions have two positive charges so are written Ca2+. How do you calculate a subscript?
- What Does The Name Surumi Mean?
- What Does Bouchon Mean In French?
- What Does Vlogger Mean | What is a Vlog? All You Need to Know about Vlogging
- What Does It Mean When A Girl Rubs Her Foot On Your Leg?
- What Does The Average Imperial Citizen Know Of The Primarchs
- What Does Ручка Mean In Russian?
- What Does Beat Mean In The Phrase Crime Beat?
- What Does Jesus Say Will Decide Our Eternal Destiny?
- What Does Causation Mean In Medical Negligence?
- What Does Überredung Mean? _ Überredung by Jane Austen
- What Ia A Flat Plane Crankshaft?
- What Does “E” Mean In Golf Scoring System?
- What Does Vci Stand For? | VC Medical Abbreviation Meaning