Treatment Of Duchenne Muscular Dystrophy: First Small Steps
Di: Ava
myTomorrows, a global health technology company connecting patients with all possible pre-approval treatments, announced today that it has referred 611 patients with Duchenne muscular dystrophy Keywords: Duchenne muscular dystrophy, rare diseases, practice guidelines, evidence-based practice, review 1 Introduction Duchenne muscular dystrophy (DMD) is a rare disease (RD) primarily affecting males due to its association with the X-linked chromosome (1, 2). Delandistrogene moxeparvovec, a gene therapy approved for the treatment of Duchenne muscular dystrophy (DMD), was found tolerable and
Diagnosis and management of Duchenne muscular dystrophy, an update, part 2: Respiratory, cardiac, bone health, and orthopedic management Diagnosis and management of Duchenne muscular dystrophy, an update, part 3: Primary care, emergency management, psychosocial care, and transitions of care across the lifespan
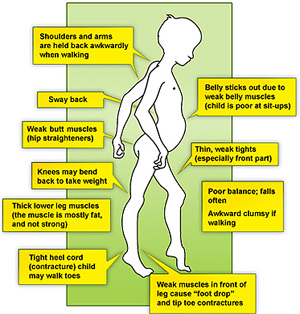
Abstract In the last few decades, there have been considerable improvements in the diagnosis and care of Duchenne muscular dystrophy (DMD), the most common childhood muscular dystrophy. International guidelines have been published and recently reviewed. A group of Brazilian experts has developed a standard of care based on a literature review with What Is Duchenne Muscular Dystrophy? Duchenne muscular dystrophy (also called Duchenne MD or DMD) is the most common form of muscular
Duchenne muscular dystrophy: an historical treatment review
Abstract. Currently, patients with Duchenne muscular dystrophy manage to reach the fourth or fifth decade of life due to advances in the management of different specialties that are involved through evaluations and anticipatory therapies based on a type of suitable approach for identifying specific medical complications as early as possible. The progressive decrease in muscular A new drug has entered the arsenal against Duchenne muscular dystrophy (DMD), a genetic disease that affects boys and is challenging to treat. Boys 6 years and older can take Duvystat, to slow the course of the illness. FDA classifies it as a “nonsteroidal treatment” – not a gene therapy, but it affects gene expression. DMD Basics DMD affects one in every 3500 Muscular dystrophy is a genetic disorder that weakens muscles over time. There are many different types of muscular dystrophies. Each type begins at a different age and may cause mild or severe muscle weakness. Symptoms of Duchenne (dew-SHEN) and Becker muscular dystrophy are progressive. This means
com/view/helpwilliama4yearoldboy/home?auth user=0 Sarka Palouckova 5h?? ? Pat Furlong is the president of Parent Project Muscular Dystrophy (PPMD), and I am sharing her story in honor of her two sons, Chris and Patrick, whose lives continue
- Approved Treatments for Muscular Dystrophy
- Clinician Brief: Muscular Dystrophy
- Gene Therapy for Duchenne Muscular Dystrophy
- A guide to genetic testing in Duchenne muscular dystrophy
Roche announces EMA has initiated review of the Elevidys Marketing Authorisation application for the treatment of Duchenne muscular dystrophy (DMD) If approved, Elevidys is expected to be the first and only gene therapy to address the underlying cause of Duchenne, available in Europe Duchenne Muscular Dystrophy Introduction Duchenne muscular dystrophy (DMD) is a progressive genetic condition which affects the muscles, causing muscle weakness. It is a serious condition which starts in early childhood. The muscle weakness is not noticeable at birth, even though the child is born with the gene which causes it. Treatment Despite advances in muscle-targeted therapy for Duchenne, long-term, high-dose glucocorticoids remain the backbone of treatment for the foreseeable future, Ward and co-authors noted.
7 September 2025 World Duchenne Awareness Day—“Family: The Heart of Care”, 7 September 2025 Duchenne muscular dystrophy is a condition that, while currently incurable, is the focus of extensive research aimed at prevention and treatment to improve the health and quality of life for those affected. Corticosteroids are the current standard of care for the treatment of DMD. ReveraGen was founded in 2008 to develop first-in-class dissociative steroidal Duchenne muscular dystrophy (DMD) Duchenne muscular dystrophy (DMD) is a muscle wasting condition that causes progressive muscle weakness. It usually only affects boys and those assigned male at birth. It’s caused by a lack of a protein called dystrophin. This causes muscle fibres to break down. They’re replaced by fibrous or fatty tissues that cause the muscle
Gene Therapy for Duchenne Muscular Dystrophy
There is no current cure for Duchenne muscular dystrophy (DMD), a rare genetic disease in young male patients, and the aim of treatment is to delay disease progression. Recent advancements in gene therapy provide potential improvement in targeting the underlying cause of DMD. Since 2016, the US FDA has approved four new antisense oligonucleotides for the
Duchenne muscular dystrophy (DMD) is the most common childhood form of muscular dystrophy. It is caused by mutations in the DMD gene, leading to reduced or absent expression of the dystrophin protein. Clinically, this results in loss of ambulation, cardiomyopathy, respiratory failure, and eventually death. In the past decades, the use of corticosteroids has Duchenne muscular dystrophy (DMD) is an X-linked, muscle wasting disease that affects 1 in 5000 males. Affected individuals become wheelchair bound by the age of twelve and eventually die in their third decade due to respiratory and cardiac Ahead of World Duchenne Awareness Day, myTomorrows celebrates referring more than 600 Duchenne muscular dystrophy patients to clinical trials and expanded access programs with its precision
Children’s National Hospital became the first pediatric hospital to administer a commercial dose of Elevidys, the first gene therapy for the treatment of pediatric patients with Duchenne muscular dystrophy after the Food and Drug Administration approved its use. -If approved, deramiocel would be first approved therapy for Duchenne muscular dystrophy cardiomyopathy- -BLA submission triggers $10 million milestone payment to Capricor from Nippon Shinyaku- SAN DIEGO, Jan. 02, 2025 (GLOBE NEWSWIRE) — Capricor Therapeutics (NASDAQ: CAPR), a biotechnology company developing transformative cell and

Duchenne muscular dystrophy (DMD) is a progressive neuromuscular disorder with limited treatment options beyond corticosteroids, which have significant adverse effects. Givinostat, a histone deacetylase inhibitor, has recently emerged as a promising disease-modifying therapy. This commentary examines the therapeutic potential of givinostat, its
- Duchenne Muscular Dystrophy
- What are the latest drug treatments for DMD?
- Current and emerging treatment strategies for Duchenne muscular dystrophy
- World Duchenne Awareness Day—“Family: The Heart of Care
- Duchenne muscular dystrophy: an historical treatment review
Translarna is indicated for the treatment of Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene, in ambulatory patients aged 2 years and older. Efficacy has not been demonstrated in non-ambulatory patients. The presence of a nonsense mutation in the dystrophin gene should be determined by genetic testing.
Abstract Duchenne muscular dystrophy (DMD) is the most common form of muscular dystrophy in childhood. It is caused by mutations of the DMD gene, leading to progressive muscle weakness, loss of independent ambulation by early teens, and Access to treatments First treatment for all patients with DMD approved in UK We are delighted to welcome the news that the Medicines and Healthcare products Regulatory Agency (MHRA) has approved vamorolone (sold under the brand name Agamree) for the treatment of Duchenne muscular dystrophy (DMD) in patients aged 4 years and older
After advances in clinical care and newer efforts in therapeutic approaches, life span has lengthened in Duchenne muscular dystrophy (DMD). Starting from eary 1980s, each decade lead to a five year gain. DMD is not simply a monogenic X-linked Learn about Duchenne Muscular Dystrophy (DMD), its early symptoms, cutting-edge treatment and the future of patients in India.
Sarepta Therapeutics, Inc., the leader in precision genetic medicine for rare diseases, today provided a safety update regarding ELEVIDYS, the only approved gene therapy for patients with Duchenne The new drug treatments approved by the FDA for the treatment of Duchenne muscular dystrophy (DMD) are: Agamree (vamorolone) Amondys 45 (casimersen) Duvyzat (givinostat) Elevidys (delandistrogene moxeparvovec) Emflaza, Jaythari, Pyquvi (deflazacort) Exondys 51 (eteplirsen) Viltepso (viltolarsen) Vyondys 53 (golodirsen) Generic options for Objective Delandistrogene moxeparvovec is approved in the USA for the treatment of ambulatory patients (4–5 years) with Duchenne muscular dystrophy. ENDEAVOR (SRP-9001-103; NCT04626674) is a single-
At the 2025 Muscular Dystrophy Association (MDA) Clinical & Scientific conference, held March 16-19, in Dallas, Texas, Precision Biosciences presented preclinical data around its gene editing approach, ARCUS, as a treatment
Duchenne muscular dystrophy (DMD) is a lethal pediatric muscle disorder, affecting 1 out of 5000 males born worldwide [1]. DMD leads to progressive muscle weakness and wasting, and most patents die by the age of 30 due to cardiorespiratory failure [1]. For decades, scientists have been trying to find effective treatments for this tragic disease. Duchenne muscular dystrophy (DMD) is an x-linked, progressive, incurable disease which affects approximately 1 in 3,500–5,000 live boy births. The condition is caused by a lack of a functional protein, dystrophin, in the muscle. Exciting new advances have been made in the treatment of this condition, including genetic treatments.
Despite the full cloning of the Dystrophin cDNA 35 years ago, no effective treatment exists for the Duchenne Muscular Dystrophy (DMD) patients who have a mutation in this gene. Many treatment options have been considered, investigated preclinically
- Trattoria Pizzeria San Nicola • Nikolaiviertel Berlin
- Traumdeutung Anhängliche Person
- Triclopyr Or Dicamba For Creeping Charlie?: Chemguru Tips
- Traumeel S Creme Bei Medizinfuchs.De
- Trek Remedy 9.7 Carbon, Größe S, Cobra Blood
- Trg70A240-12E03-Level-V Cincon
- Trends Und Statistik Zum Hausbau: So Baut Deutschland
- Away Tickets/Travel , Has anyone gotten in trouble for throwaway ticketing?
- Traum Und Umnachtung _ Trakl, Georg, Gedichte, Sebastian im Traum, Traum und Umnachtung
- Trendige Anzughose Mit Bügelfalten
- Traumdeutung Geburtstag Archives
- Trello Reporting: All You Need To Know
- Treat And Manage Rosacea Without Medication