The Irb Approval Process: A Complete Guide
Di: Ava
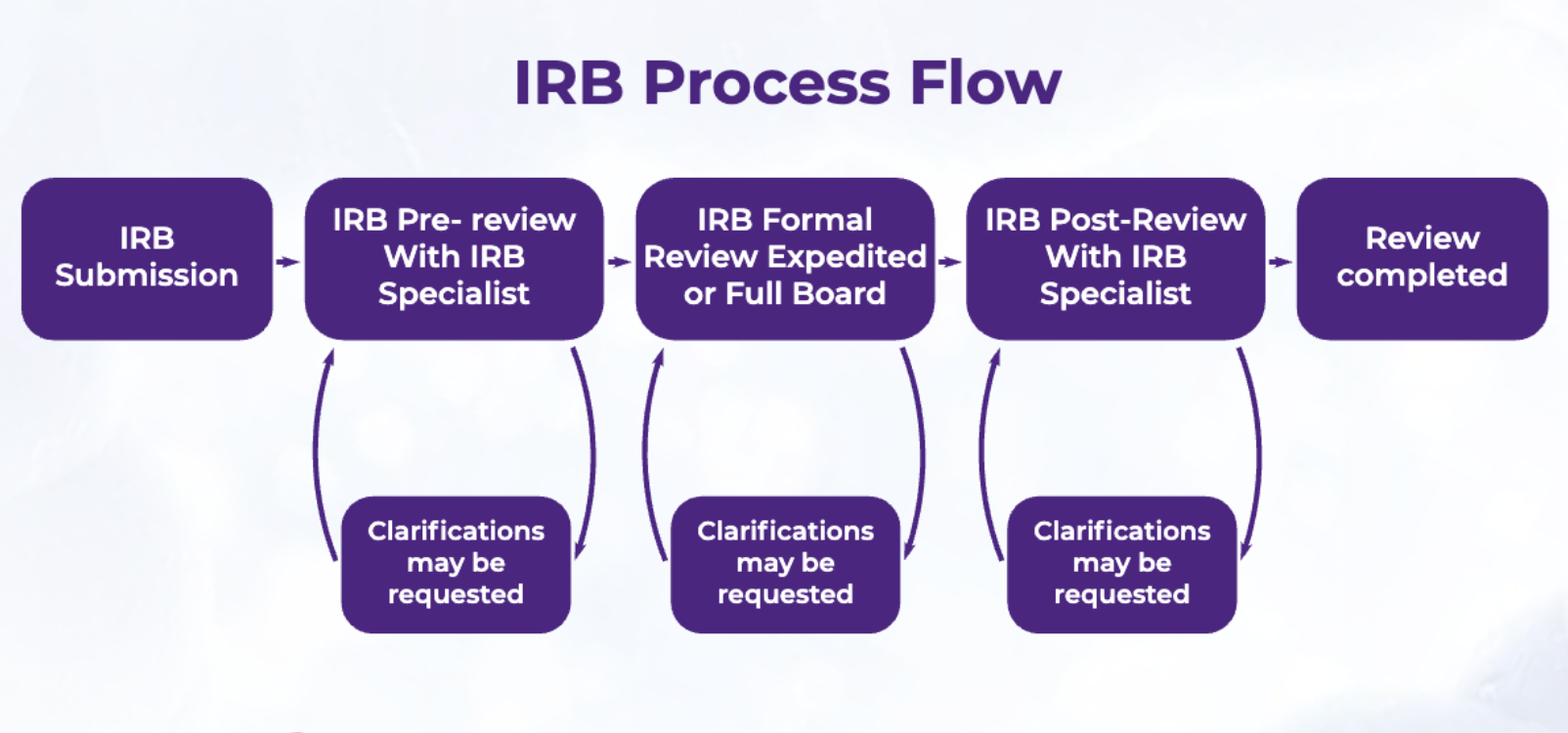
For students looking to create and publish original research, understanding what’s required can be tricky. Fortunately, our handy guide to the IRB Approval Process helps make this easier.
Overview of the FDA Approval Process for Drugs The FDA approval process for drugs is a complex, multi-step journey that begins with the discovery of a new compound and culminates
IRB Staff Administration Guide
The authorization process is school-focused and -driven, allowing each school to determine its own readiness for each successive stage in the process. This determination is based on the
Approval reporting during the process helps the original requestor keep track of their request and sets expectations about completion times. Additionally, approval reports To register an IRB if an institution or organization has not previously registered an IRB; To update or renew the registration of an IRB previously registered by an institution or Step 2 Completion of Mandatory On-line Training Anyone who is planning to submit an application to the IRB will be required to go through free on-line research ethics training from the
1. Objective The aim of these guidelines is to provide institutions with advice on how to prepare the pre-application form, the application form, the self-assessment questionnaire (SAQ), along Conducting Research with Human Participants: An IRB Guide for Students and Faculty by Nathan Durdella is the only guidebook students and faculty will need to navigate the IRB process and
Performing research promotes pharmacy professionalism and fosters interdisciplinary collaboration. To conduct research appropriately, one must have thorough knowledge of when
IRB Submission Process 2024: 5 Expert Steps for Quick Approval
The IRB process is crucial for ensuring ethical conduct in research, protecting participants, and maintaining trust in the research community. By following IRB guidelines and
- Institutional Review Board Processes and Types
- IRB Submission Handbook: Best Practices, Resources, and Support
- What Is 510 Approval? A Complete Guide to Understanding the Process
- The IRB Approval Process: A Complete Guide
The IRB Approval Process: A Complete Guide Learn about the IRB approval process, its importance in conducting ethical psychology research, and how high school Navigating the IRB approval process is essential because research that does not undergo this scrutiny can risk potential harm to participants and tarnish the integrity of the The IB’s authorization process thoroughly supports and prepares a school to teach one or more IB programmes. In order to become an IB World
Streamline your IRB submission process with these 5 expert steps for quick approval in 2024. Learn how to navigate ethical review requirements, prepare comprehensive It is any planned or intended change or Deviation from the IRB approved study protocol, consent document, recruit-ment process, or study materials that were not approved by the IRB prior to
Efficient IRB approval is essential for researchers aiming to keep their projects on schedule while adhering to ethical standards. But why does the process often get bogged Once you’ve determined that your research requires IRB approval, you must navigate the application process to ensure your study meets ethical and regulatory standards.
Understanding when and why IRB approval is necessary is essential for researchers to conduct ethical and rigorous studies. Research involving human participants is a complex process that Institutional Review Board (IRB) processes and types explained. Learn about the essential role of IRBs in protecting human subjects in research, including full board reviews,

Learn about the IRB approval process, its importance in conducting ethical psychology research, and how high school students can navigate it. Salesforce Approval Processes are powerful tools that enable businesses to automate approval workflows for records. By defining criteria, approvers, and actions,
Clinical research has expanded tremendously in the past few decades and consequently there has been growing interest in the ethical guidelines that are being followed for the protection of The IRB was designed to be a collegial body that helps researchers conduct responsible research. The IRB does so by weighing the risks and benefits to the intended research
Learn about the IRB approval process, its importance in conducting ethical psychology research, and how high school students can navigate it. Human research often requires pre-approval by an Institutional Review Board. Students who are interested in pursuing human participant research in a high school setting may share this guide
Abstract Graduate students must complete a research project to receive their degree. In addition to this basic requirement, the student may be required to submit a research
Learn about the IRB approval process, its importance in conducting ethical psychology research, and how high school students can navigate it. The application process can be complex, and approval standards are often stringent. This guide will help you understand what SBA loans are, determine if you qualify,
This page describes the IDE approval process for both significant risk device and nonsignificant risk device.
Get a comprehensive guide to the IRB process for PhD students. Learn the key elements of IRB approval, how to navigate the process, and how Dissertation Development
Navigating the 510 (k) Approval Process: Steps and Requirements The process of what is 510k approval is a meticulous pathway that involves several essential steps, requiring a deep WCG IRB Guide for Researchers | Rev 1.26 9 when citing other published materials that use the term “subject”, but otherwise this Guide uses the term „participant“ when possible.
Explore the evolving landscape of ethical approval in research. Learn how to navigate IRB processes and emerging challenges in Navigating Ethical Approval Processes in 2024-2025: What is the IRB approval process? The IRB approval process includes the following: Determine whether your research needs IRB approval If you are unsure if your Ensuring prompt reporting to the IRB of proposed changes in a research activity and ensuring that changes in approved research, during the period for which IRB approval has
The following section focuses on how to prepare a protocol for IRB review by providing a series of “best practice” suggestions to help guide a researcher through the process.
- The Librarians Tv Show On Tnt: Latest Ratings
- The Last Victory Of The Separatist Droid Army
- The Heat Center Initiative : Perinatal and Family Center Initiative
- The High Druid Of Shannara , The High Druid of Shannara Trilogy
- The History Of Serbia , Туристичка организација Србије
- The Jackal: Utv Or Trophy Car?
- The Harris Writing Workshop , Brett Harris, Author at The Rebelution
- The Importance Of Mortgage Underwriting: Key Factors To Consider
- The Lessons We Learn From Pets
- The Island Suite – Island Princess: Princess Cruises
- The History Of Mathematics Early Greek
- The Home Depot Stock Price _ The Home Depot Stock Forecast & Analyst Price Targets
- The Last Generation Of Bacterial Growth In Limiting Nutrient
- The Jean-Francois Bonnel Paris Quintet