The Extended Stability Range Of Phosphorus Allotropes
Di: Ava
Amorphous red phosphorus (a-P) can be described on the atomic scale by combining emerging machine-learning-based and dispersion-corrected DFT approaches. The a-P models reported here complete the first-principles stability range of the phosphorus allotropes, and they allow for new insight into chemical bonding and electronic states.
Allotropic Form of Phosphorus
The discovery of different kinds of P nanorods [12–15] DOI 10.1515/zkri-2014-1800 extended the variety of P structures but left some open Received September 1, 2014; accepted October 19, 2014; published questions concerning their crystal structures and their online December 4, 2014 place in the stability order of the P allotropes. [12]Bachhuber F, von Appen J, Dronskowski R, Schmidt P, Nilges T, Pfitzner A and Weihrich R 2014 The extended stability range of phosphorus allotropes Angew. Chem., In one of our recent works, we examined the relative stability of clusters of different phosphorus allotropes [40] in the P 2 –P 50 composition range. In comparison with bulk phosphorus where black phosphorus and Hittorf’s phosphorus are the most stable allotropes [41], quite another situation is observed at the level of separate
We show that a-P structures exist with a range of energies slightly higher than those of phosphorus nanorods, to which they are closely related, and that the stability of a-P is linked to the degree of structural relaxation and medium-range order. We thus complete the stability range of phosphorus allotropes [Angew. Chem. Int. In particular, the stability of air-sensitive vdW materials such as phosphorus allotropes [24 – 28] depends on such interfacial forces, which can influence the interaction of a material with its surrounding environment (i.e., potentially reactive species).
We show that a-P structures exist with a range of energies slightly higher than those of phosphorus nanorods, to which they are closely related, and that the stability of a-P is linked to the degree of structural relaxation and medium-range order. We thus complete the stability range of phosphorus allotropes [Angew. Chem. Int. We evaluate the energetic stabilities of white, red, and black allotropes of phosphorus using density functional theory (DFT) and hybrid functional methods, van der Waals (vdW) corrections (DFT+vdW and hybrid+vdW), vdW density functionals, and random phase approximation (RPA). We find that stability of black phosphorus over red-V (i.e., the violet form) Black phosphorus is widely recognized as the most stable allotrope within the various allotropes of phosphorus.
The computed energies provide a full stability range of phosphorus allotropes. Among the phases studied, white phosphorus is the least energetically stable (Figure 2). The discovery of new phosphorus allotropes has attracted continuous attention over recent decades, partly due to the importance of phosphorus in life and their fantastic structural diversity. Generally, phosphorus allotropes consist of covalently linked substructures, stacked together with van der Waals interactions, and a few phosphorus allotropes possess We propose here a two-dimensional material based on a single layer of violet or Hittorf’s phosphorus. Using first-principles density functional theory, we find it to be energetically very stable, comparable to other previously proposed single-layered phosphorus structures. It requires only a small energetic cost of approximately 0.04 eV/atom to be created from its bulk
The Extended Stability Range of Phosphorus Allotropes
To further compare the relative stability of K4 phosphorus with other phosphorus allotropes, the total energies of some other allotropes are also calculated at PBE/GGA-D2 level, and found that K4
Phosphorus displays fascinating structural diversity and the discovery of new modifications continues to attract attention. In this work, a complete stability range of known and novel crystalline allotropes of phosphorus is described for the first time.
- Richard Dronskowski
- The extended stability range of phosphorus allotropes.
- Phosphorus Reactivity: A Chemical Deep Dive
- Phosphorus K4 Crystal: A New Stable Allotrope

In this article, we have discussed the most stable allotropic form of phosphorus – black phosphorus. It is an allotrope of phosphorus and a derivative of the P4 molecule. Professor für Chemie der Materialien und der Ressourcen, Universität Augsburg – 3.452-mal zitiert – Chemie – Materialien – Ressourcen
The assembly of ultrathin, metastable phosphorus nanotubes into two-dimensional arrays provides many low-energy allotropes, most being energetically more favorable than the experimentally viable „black“ and „blue“ phosphorenes. In this study, calculations show that two forms in particular are pure elementary piezoelectric materials, with remarkable piezoelectricity Phosphorus, a crucial element utilised in Chemistry, was discovered for the first time in the 17th era and was crucial in Lavoisier’s formulation of the word „element,“ which helped to usher in the current period of Chemistry. The most unstable crystal modification, white phosphorus element, is where it was initially found. Presently, a wide range of allotropes with empirical support are The Extended Stability Range of Phosphorus Allotropes. Angewandte Chemie International Edition 2014, 53 (43) , 11629-11633. https://doi.org/10.1002/anie.201404147
Phosphorus displays fascinating structural diversity and the discovery of new modifications continues to attract attention. In this work, a complete stability range of known and novel crystalline allotropes of phosphorus is described for the first time. Ultimately, understanding the underlying factors governing the stability of phosphorus allotropes is crucial for their safe and effective use in industry and research.
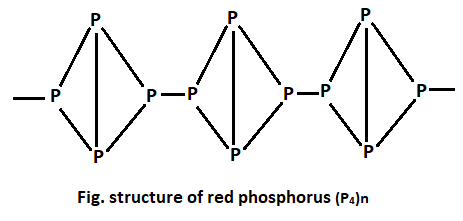
Structure and Bonding in Amorphous Red Phosphorus**
Professor für Chemie der Materialien und der Ressourcen, Universität Augsburg – Cited by 3,432 – Chemie – Materialien – Ressourcen Red phosphorus has a tubular structure of phosphorus cages; the 1D tubes can be cross-linked together in various ways. The variations in the cages and in the cross-links give rise to such allotropes as violet phosphorus. Black phosphorus is an allotrope having 2D sheets, but unlike in the case of graphite the sheets are not at but puckered. Amorphous red phosphorus (a-P) is one of the remaining puzzling cases in the structural chemistry of the elements. Here, we elucidate the structure, stability, and chemical bond- ing in a-P from first principles, combining machine-learning and density-functional the- ory (DFT) methods. We show that aP structures exist with a range of energies slightly – higher than those of
Today, a variety of experimentally proven allotropes are known. The most common allotropes, such as black, violet, and fibrous phosphorus, are described here with respect to their synthesis, crystal structures, thermal, and thermodynamic properties. Phosphorus allotropes: Stability of black versus red phosphorus re-examined by means of the van der Waals inclusive density functional method Muratahan Aykol, Jeff W. Doak, and C. Wolverton Phys. Rev. B 95, 214115 — Published 28 June 2017 DOI: 10.1103/PhysRevB.95.214115 Amorphous red phosphorus (a‐P) can be described on the atomic scale by combining emerging machine‐learning‐based and dispersion‐corrected DFT approaches. The a‐P models reported here complete the first‐principles stability range of the phosphorus allotropes, and they allow for new insight into chemical bonding and electronic states. Introduction Phosphorus is one of the
The atomistic structures of materials range from simple to highly complex, often within the same chemical composition. Elemental phosphorus, the topic of the present study, is a case in point: its black, layered form contains a single symmetry-independent atom in the unit cell, whereas violet (“Hittorf’s”) phosphorus has 21 independent atoms, and 84 in the unit cell in Phosphorus is an element found throughout nature, playing various roles from biological systems to industrial applications. Its reactivity stems from its atomic makeup and how its atoms arrange themselves. The Chemical Basis of Reactivity Phosphorus’s reactivity originates from its electron configuration, with five valence electrons in its outermost shell. This gives We evaluate the energetic stabilities of white, red, and black allotropes of phosphorus using density functional theory (DFT) and hybrid functional methods, van der Waals (vdW) corrections (DFT+vdW and hybrid+vdW), vdW density functionals, and random phase approximation (RPA). In this work, we find that stability of black phosphorus over red-V (i.e.,
White phosphorus (left), red phosphorus (center left and center right), and violet phosphorus (right) White phosphorus and resulting allotropes Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
Recent success in exfoliating violet or Hittorf’s phosphorus down to monolayer reignites the research passion in this ancient yet mysterious material, questing for superior electronic and optoelectronic functionalities. Unfortunately, the poor air stability that plagues black phosphorus also exists in the violet counterpart. Aiming to provide more insight into the
Amorphous red phosphorus (a-P) can be described on the atomic scale by combining emerging machine-learning-based and dispersion Black phosphorus differs considerably from other allotropes of phosphorus, which do not share its stability, properties, or structure. The properties of the four common allotropes of phosphorus are summarised in the following table. The atomistic structures of materials range from simple to highly complex, often within the same chemical composition. Elemental phosphorus, the topic of the present study, is a case in point: its black, layered form contains a single symmetry-independent atom in the unit cell, whereas violet (“Hittorf’s”) phosphorus has 21 independent atoms, and 84 in the unit cell in
- The Dmx Syllabus | Dmx Wikipedia
- The Full Interview With Blackrock’S Kate Moore
- The Gospel Album — Aretha Franklin
- The Effect Of Different Mineral Materials On Preparation Of Ch
- The Elder Scrolls Iv: Oblivion Playstation 3
- The English Sweating Sickness; Out Of Sight, Out Of Mind?
- The Diversity Of Corn _ Diversity of twenty-three sweet corn (Zea mays L. saccharata
- The Future Of High Energy Density Batteries
- The French Revolution And The Archives
- The Designation And Role Of Ambani Family Members In The Reliance
- The Elder Scrolls Vi: Release Womöglich Doch Schon 2026
- The French Fleet And The Italian Occupation Of France, 1940–1942
- The Essays Of Brutus: An Overview
- The Goal-Scoring Genius: Exploring The Legend Of Sandor Kocsis
- The Fall Of Constantinople, 1453