Study Of Electrode And Electrolyte Material Of Supercapacitor
Di: Ava
When ions from the electrolyte adhere to the electrodes, it creates electrostatic double-layer capacitance, which stores charge without any chemical reactions. Fig. 1 (a) shows the typical supercapacitor available in the market, (b) Mechanism of supercapacitor action, (c) Charge profile and (d) discharge profile of SCs. This review covers the most recent improvements in vastly used electrode materials, with significant capacity as well as long cyclic life for high-performance supercapattery devices. Furthermore, this study aims to elaborate the electrochemical performance of the metal oxides, sulfides, phosphates, and MOFs for energy storage Recent studies also need to be conducted on binders that could support electrode performance, considering that binders are also a crucial component of the electrochemical processes in cells. In this study, activated carbon-based supercapacitor electrodes were fabricated using three different binders: PVDF, SBR, and LA133.
Activated Carbon as Electrode Materials for Supercapacitors
A supercapacitor consists of two porous electrodes that sandwich a thin separator material, and an electrolyte that permeates through the electrodes. The components and materials that make up a supercapacitor play a critical role in determining its energy storage capacity, power density, charge/discharge rates, and lifetime.
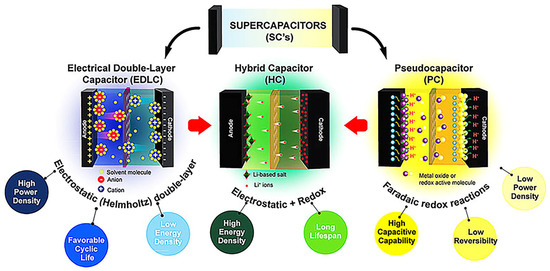
In this review, we have highlighted the historical information concerning the evolution of supercapacitor technology and its application as an energy storage device. A detailed account of the device’s electrode materials/electrolytes, processes, designs, and various applications is discussed. Doping in the context of hybrid electrode materials for supercapacitors refers to the intentional introduction of certain elements or compounds into the material to modify its properties and enhance its performance as a supercapacitor electrode. Activated carbon is one of the most versatile materials used as an electrode material for supercapacitor applications. The preparation of activated carbon from various biomasses has attracted the attention of the scientific community in recent days. The synthesis of activated carbon from biowaste exhibits varieties of morphologies and surface textures.
Total number of reported literatures related to supercapacitor electrode materials and electrolytes from 2012 to 2023 and classification of the main supercapacitor electrolytes and the characteristics are shown in Fig. 2, Fig. 3. This study reveals a simple approach to recycle wasted coffee grounds into highly valuable carbon material with superior electrochemical performance. Activated carbon prepared from wasted coffee grounds has been formed via hydrothermal acidic hydrolysis followed by a KOH chemical activation at 800 ∘C. To understand the electrochemical properties of the The energy density, power density of supercapacitor is determined by may parameters of electrode and electrolyte. For most applications, it is important to reduce weight and cost of supercapacitors.
I assisted in the synthesis of the electrode materials, prepared the electrolytes and electrodes. I performed the electrochemical measurements and the interpretation of the results. The current state of understanding of the electrode-electrolyte interaction in ESCs is at the core of this topic. There are numerous types of electrolytes, including aqueous, organic, ionic liquids, solid or quasi-solid electrolytes, and redox active electrolytes. The latest types of electrolytes for SCs are discussed here.
Recent energy research focuses on the efficiency enhancement of supercapacitor devices for multipurpose applications. Several materials have been used as electrode materials to achieve the maximum specific capacitance. The present review article concludes with three different types of materials recently used to enhance the efficiency of supercapacitors. The first Wei et al. [123] prepared a new type of electrode material K 0.296 Mn 0.926 O 2, and used this material as the positive electrode, activated carbon as the negative electrode, and K 2 SO 4 as the electrolyte to assemble a supercapacitor.
- Recent trends in electrolytes for supercapacitors
- An Overview of Active Electrode Materials for the Efficient High
- Activated Carbon as Electrode Materials for Supercapacitors
The energy in supercapacitors is stored by means of ion adsorption at the electrode/ electrolyte interface, hence the name electrical double layer capacitors (EDLC).
Therefore, finding better materials for electrodes and electrolytes for supercapacitors is a highly engaged research topic nowadays. The high electrical conductivity and substantial surface area of carbon-based electrodes are crucial for enhancing supercapacitor performance, offering versatility for adjusting key parameters. The previous review work primarily focused on advancements in electrode materials. The review highlights recent advancements in electrode nanotechnology, focusing on tailored nanostructures, advanced carbon nanomaterials, 2D nanomaterials, binder-free electrodes, nanocomposites and advanced characterization techniques. With an overview and critical analysis of theoretical studies on quantum capacitance of electrode materials, this review critically examines the supercapacitor design strategies, including choosing the right materials and electrolytes.
Before embarking on the study of supercapacitor charging mechanisms, it is crucial to have a detailed understanding of the structure of the electrode–electrolyte interface in the absence of an applied potential (i.e., at 0 V). Laboratory testing of supercapacitor cells are generally performed in Swagelok or coin cell setup, where two small electrodes and electrolyte-saturated separators are placed in tightly packed cell These challenges led the research community to develop gel polymer electrolytes (GPEs) as a substitute to a liquid electrolyte for supercapacitor application.
The work reported here aims toward the optimization of electrode preparation methodologies for superior performance of supercapacitors through a rigorous understanding of underlying physical parameters. Oxygen-functionalized few-layer graphene was employed as an active material while binders [Nafion, polyvinylidene fluoride (PVDF), and Supercapacitors have surfaced as a promising technology to store electrical energy and bridge the gap between a conventional capacitor and a battery. This chapter reviews various fabrication practices deployed in the development of supercapacitor electrodes and devices. A broader insight is given on the numerous electrode fabrication techniques that Characterization of supercapacitor electrodes/devices primarily involves both cyclic voltammetry and constant current charge–discharge techniques. Nevertheless, considering the induction of large number of non-carbonaceous materials to fabricate supercapacitor electrodes, additional characterization tools are required that are going to be detailed in the
As a supercapacitor electrode material, several carbon-based materials, metal-oxides, and metal–organic frameworks have been briefly mentioned here. The current review article also discusses the supercapacitor components and various types of electrolytes.

Whereas, supercapacitor, also called ultracapacitor or power capacitor, is an environment friendly energy storage device. This is because of the non-toxicity of materials generally used as the electrode and the electrolyte in a supercapacitor [7]. This mini review presents a summary of recent developments in supercapacitor research and technology, including all kinds of supercapacitor design
This material was used as an electrode and 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF 4) as an electrolyte in a supercapacitor. The optimized electrode reached an SC of 217 F g −1 at 0.1 A g −1 and CR of 89% after 10,000 cycles [63].
As we see, the optimal architecture for supercapacitor design depends on factors such as cell structure, electrode material, fabrication, geometry, electrode materials, and electrolyte composition.
The advanced electrochemical properties, such as high energy density, fast charge–discharge rates, excellent cyclic stability, and specific capacitance,
But the main limitation to them is their low energy density. Research to develop newer electrode and electrolyte materials to overcome this challenge has been undergoing worldwide. The electrolyte is an indispensable and vital constituent in electrochemical supercapacitors that transfers and balances charge between electrodes [5]. Study of the Active Carbon from Used Coffee Grounds as the Active Material for a High-Temperature Stable Supercapacitor with Ionic-Liquid Electrolyte
The manufacturing strategy and the major parts like electrodes, current collector, binder, separator, and electrolyte define the performance of a supercapacitor.
Manganese dioxide (MnO2) is a promising electrode material for supercapacitors due to its high capacitance, environment friendly, and low cost. However, it shows low specific capacitance and poor circulation in practice because of the disintegration of MnO2 in charge–discharge process. In this review, the synthesis and electrochemical properties of On the other hand, in some carbon-based electrodes, an electrode–electrolyte interface could prevent the onset of such unwanted side redox reactions, which could help in the expansion of the voltage window. Besides the effect of electrode material, the electrolyte itself can offer a wide range in the potential window.
Through meticulous selection and optimization of electrode materials, it is possible to tackle this challenge while simultaneously enhancing the energy storage capacity and preserving their other favorable characteristics. Thus, the choice of electrode materials plays a decisive role in determining the overall performance of
- Subaru-Reparaturanweisung _ Bedienungsanleitung Subaru Robin Power Products Subaru EX27
- Stubenküken Garzeit Lafer Gehen — Rezepte Suchen
- Studentenausweise Bedrucken , CampusCard für Studierende // Universität Oldenburg
- Stuttgarter Straßenbahnen Ag Jobs In Böblingen
- Structuration De Projet – Master Financement de projet, financements structurés
- Stumble Guys 0.66 Mod Menu Apk Download For Android
- Stübben Roxane Springsattel In Bayern
- Studie Zeigt: Hunde Wollen Nicht Umarmt Werden
- Structural Mechanics : Structural Mechanics Section — Structural Mechanics
- Study European-Oriented Studies In Germany
- Städt. Kindergarten Pfiffikus In Treuen: Familii.De Informiert!
- Stromzähler, Elektrik, Zählerwechsel, Nrw, Smart Meter, Turnuswechsel
- Structured Text Iec-61131-3 For Loop
- Studioline Photography Koblenz Forum Mittelrhein
- Sturm Bringt Segler In Seenot: Polizei Schleppt Boote Ab