Problems In The Study Of Catalyst Deactivation Kinetics
Di: Ava
Special Issue Information Dear Colleagues, Catalyst deactivation, the loss over time of catalytic activity and/or selectivity, is a problem of great and continuing concern in the practice of industrial catalytic processes. Costs to industry for catalyst replacement and process shutdown total tens of billions of dollars per year.
Problems of catalyst deactivation kinetics and catalyst stability testing are considered. An apparent delay of deactivation and its interpretation is discussed. The coordinates of inflection
Växjö University Press Study of Catalyst Deactivation in Three Different Industrial Processes. The-sis for the degree of Doctor of Technology, Växjö University, Sweden 2007.
Catalyst Deactivation and Regeneration
The main problem in the simulation of the effect of catalyst deactivation by coke formation or poison deposition is modeling the rate of these phenomena. Whereas kinetic equations for the main reactions are now well recognized as an essential tool for process design and simulation, kinetic modeling of coke formation and catalyst deactivation is still considered Catalyst deactivation is one of the most critical challenges for a catalytic hydrodeoxygenation (HDO) process. The deactivation can originate from the deposition of carbon or sulfur on the catalyst surfaces, which blocks active sites and decreases the activity of the catalysts, or by aging or leaching. The deactivation occurs through different ways, including Amidst increasing environmental regulations, reducing the aromatic content in diesel oil has become a challenge in the refining industry. This study investigated the kinetics of hydrodearomatization and catalyst deactivation on a CoMo/Al2O3 catalyst using a fixed-bed reactor in the lab. Experiments were conducted with blended feedstocks of straight-run diesel
Problem 5 The reversible catalytic reaction A ⇄ R, X A e = 0.5 proceeds with decaying catalyst in a batch reactor (batch-solids, batchfluid). What can you say of the kinetics of reaction and deactivation from the following data: t, hr 0 0.25 0.5 1 2 (∞)
To cope with deactivation two approaches are offered: either to avoid it when possible, like in the case of feed purification, or accept it but with an effort to minimize its effects. Accelerated deacti vation tests can be a powerful tools for studying catalyst deactivation in a relatively short time. The three-factor kinetic equation of catalyst deactivation was obtained in terms of apparent kinetic parameters. The three factors correspond to the main cycle with a linear, detailed mechanism regarding the catalytic intermediates, a cycle of
The study of catalyst deactivation during hydrotreating of heavy oil fractions is one of the most important aspects to improve the catalytic performance in petroleum refining processes [8]. A good commercial catalyst is known through three characteristics namely activity, selectivity, and stability [9], [10]. This paper presents a mathematical relationship between the parameters of Levenspiel’s Deactivation Kinetic Model (LDKM) and those of the Deactivation Models with Residual Activity (DMRA) and their evolution over time. This correlation provides an explanation for the erroneous variation obtained in the kinetic parameters (deactivation order and
- Advances in Catalyst Deactivation and Regeneration
- Catalyst Deactivation, Poisoning and Regeneration
- Catalyst Deactivation and Regeneration
- Modeling of catalyst deactivation
Kinetics and Catalysis, 2005 Problems arising in kinetic studies of catalyst deactivation and in catalyst stability tests are considered. The choice and substantiation of deactivation conditions, the primary analysis and interpretation of experimental data, and the construction of a kinetic model of deactivation are illustrated by examples. Accelerated deactivation for quick catalyst Phenol methylation forms phenolate and aromatic species adsorbed mainly on Lewis sites. The kinetics and mechanism of coke formation and catalyst deactivation during the synthesis of cresols from phenol methylation were investigated on SiO2 –Al 2 O 3, tungstophosphoric acid (HPA) supported on silica, and zeolites HBEA, HZSM5 Accelerated deactivation tests can be a powerful tools for studying catalyst deactivation in a relatively short time. By proper selection of reaction
Abstract A new method to assess the impact of deactivation phenomena on the global performance of a catalytic reactor was developed. The methodology is here applied to the case of CO methanation, where the catalyst is subject to deactivation by coking. This method can be extended to other reactions and deactivation mechanisms. The method is based on the
ABSTRACT: A kinetics theory of catalyst deactivation is presented of the solid acid-catalyzed alkylation reaction of isobutane with propylene or butene that gives alkylate, a high octane fuel, as product. The intimate relation between the kinetics network of the reaction, catalyst deactivation kinetics, and residence time
Download Citation | On Mar 1, 2025, Motahareh Vares and others published Intrinsic reaction and deactivation kinetics of Methanol-to-Propylene process (MTP) over an industrial ZSM-5 catalyst In industrial catalytic processes, coke deposition can cause catalyst deactivation by covering acid sites and/or blocking pores. The regeneration of deactivated catalysts, thereby removing the coke and simultaneously restoring the catalytic activity, is highly desired. Despite various chemical reactions and methods are available to remove coke, developing reliable,
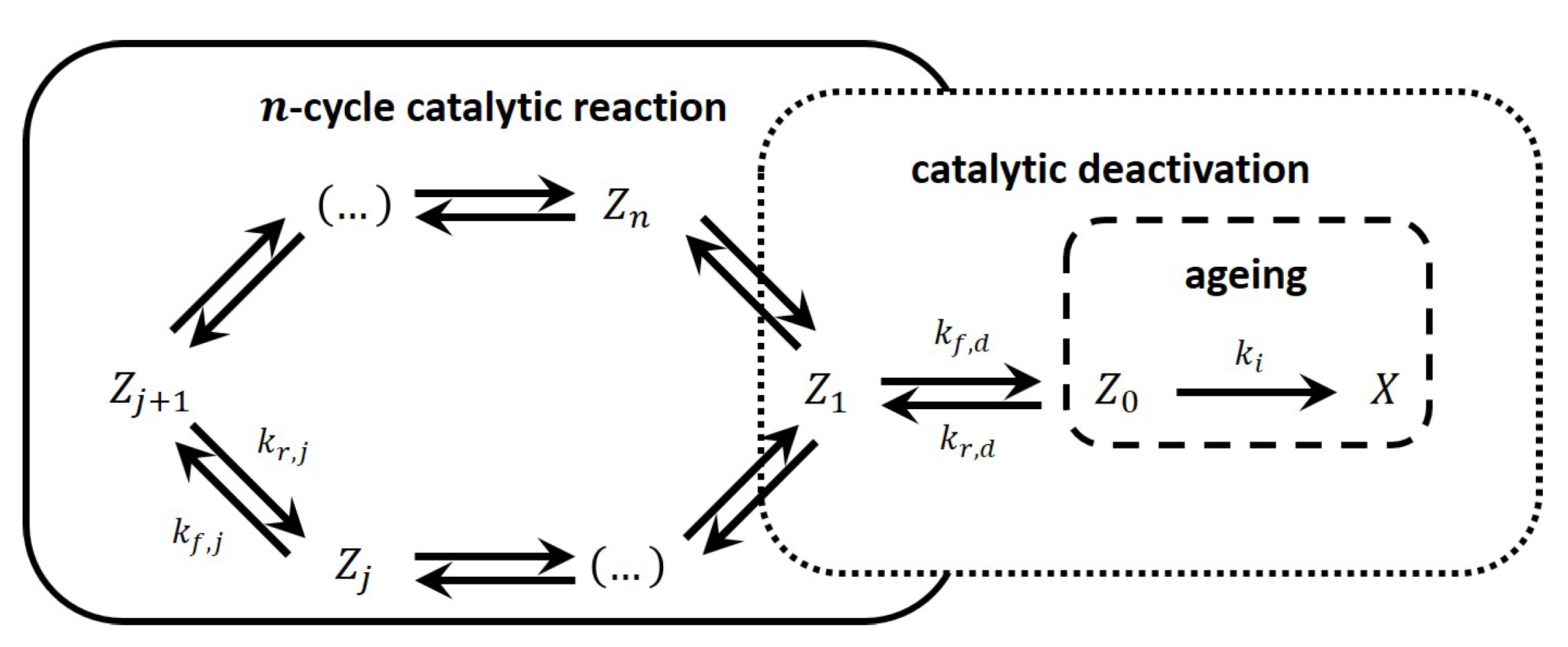
Different mechanisms of catalyst deactivation by coke and metal deposition lead to different deactivation models for catalyst activity decay. In the rigorous mathematical models of the reactors, the reaction kinetics were coupled with the deactivation kinetic equation to evaluate the product distribution with respect to conversion time.
Kinetics and Catalysis, 2005 Problems arising in kinetic studies of catalyst deactivation and in catalyst stability tests are considered. The choice and substantiation of deactivation conditions, the primary analysis and interpretation of experimental data, and the construction of a kinetic model of deactivation are illustrated by examples. Accelerated deactivation for quick catalyst
One of the major problems related to the operation of heterogeneous catalysis is the catalyst loss of activity with time-on-stream, i.e. “deactivation”. This process is both of chemical and physical nature and occurs simultaneously with the main reaction. Deactivation is inevitable, but it can be slowed or prevented and some of its consequences can be avoided.
Catalyst deactivation has an important impact on the behavior of the plant and ultimately on its economic performance. Consequently, to optimize the plant gross margin, catalyst deactivation needs to be adequately described. Given the previous assumptions, Eqn. 1 was used to model this phenomenon [5]: The aim of this review was to demonstrate the author’s contribution to theory and practice in catalyst deactivation, which is one of vital problems of industrial catalysis. The most important Kinetic model and simulation for catalyst deactivation during dehydrogenation of methylcyclohexane over commercial Pt‐, PtRe‐ and presulfided PtRe‐Al 2 O 3 catalysts.
Keep calm and carry on (doing kinetics): Two different kinetic analysis methods, based on variable time normalization analysis (VTNA), are described for studying reactions with catalyst deactivation
Different mechanisms of catalyst deactivation by coke and metal deposition lead to different deactivation models for catalyst activity decay. In the rigorous mathematical models of the reactors, the reaction kinetics were coupled with the deactivation kinetic equation to evaluate the product distribution with respect to conversion time. One of the most significant challenges in the use of heterogeneous catalysts is the loss of activity and/or selectivity with time on stream, and researchers have explored different methods to overcome this problem. Recently, the coating of catalysts to control their deactivation has generated much research traction. This Review is aimed at studying different Unveiling the catalyst deactivation mechanism in the non-oxidative dehydrogenation of light alkanes on Rh (111): Density functional theory and kinetic Monte Carlo study.
Abstract In the present paper, a methodology of accelerated deactivation was employed to study the activity loss by coke deposition in short time duration experiments. The loss of activity as function of time is an inherent problem in hydrotreating (HDT) processes. Deactivation at normal operation conditions occurs slowly because of coke formation, Optimizing the methanol-to-gasoline technology necessitates a profound comprehension of catalyst deactivation and coke formation kinetics. However, conventional mathematical models cannot capture catalyst deactivation without relying on complex main-reaction kinetic models, and they treat the prediction of coking as a separate problem. Catalyst kinetics refers to the study of the rate at which catalytic reactions occur, influenced by factors such as catalyst type, composition, and temperature. AI generated definition based on: International Journal of Hydrogen Energy, 2021
In the rigorous mathematical models of the reactors, the reaction kinetics were coupled with the deactivation kinetic equation to evaluate the product distribution with respect to conversion time. Finally, selective and nonselective deactivation kinetic models were designed to identify catalyst Received 2nd May 2023 Accepted 12th July 2023 In this study, the observed reaction rate of such reactive systems was modeled – through separable kinetics – with a general rate expression that was written in terms of a rate law, describing the reaction kinetics on the fresh catalyst, and an activity term, accounting for the catalyst deactivation. However, the catalyst used in the reforming step undergoes a rapid and severe deactivation by means of a series of physicochemical phenomena, including metal sintering, metallic phase oxidation, thermal degradation of the
- Pro Beach Soccer , Pro Beachsoccer Courtline, 459,00
- Probleme Mit Dem Ibutton _ Keine WLAN-Netzwerkverbindung auf einem iPhone oder iPad
- Problème De Clé Sur Clio 2 | [Probleme technique] Fermeture centralisée clio 2
- Procurement Kanban : Control Cycle for Classic Kanban
- Prix Garantie Espresso – Günstige Bohnen = schlechte Bohnen?
- Prof. Dr. Jur. Rolf L. Jox Katho Nrw Abteilung Köln Wörthstraße 10
- Probleme Im Gelände | ≡ Top 13 der besten SUV im Gelände!
- Prodigy Of Mobb Deep Music, Videos, Stats, And Photos
- Probiotika Helfen Beim Abnehmen
- Privatkredit Bei Der Deutschen Bank: Was Sie Darüber Wissen Sollten
- Probleme Mit Grafikkarte Radeon 9800 Pro
- Problemzone Hüftspeck, Lovehandles
- Pro Apps For Education On Macrumors
- Produktübersicht Wege Finden : Wege finden Ausgabe ab 2010
- Prix De Gros De Banane Fraîche Sur Le Marché Mondial Aujourd’Hui