Prediction Of Amino Acid Sequence From Structure
Di: Ava
SSEmb is a multi-modal machine learning model that predicts how changes in a protein’s amino acid sequence affect its function by combining information from a multiple sequence alignment and the Abstract The prediction of protein three-dimensional structure from amino acid sequence has been a grand challenge problem in computational biophysics for decades, owing to its intrinsic scientific interest and also to the many potential applications for robust protein structure prediction algorithms, from genome interpretation to protein function prediction. More recently, the inverse
Why predict protein structure? Problem definition Given the amino acid sequence of a protein, predict its three-dimensional structure Proteins sample many structures. We want the average structure, which is roughly what’s measured experimentally. Scratch Protein Predictor – (Institute for Genomics and Bioinformatics, University California, Irvine) – programs include: ACCpro: the relative solvent accessibility This hypothesis sparked a five decade quest to be able to computationally predict a protein’s 3D structure based solely on its 1D amino acid sequence as a complementary alternative to these expensive and time consuming experimental methods.
3-D Structure Prediction This is collection of freely accessible web tools, software and databases for the prediction of protein 3-D structure. Template-base modeling Meta servers Sequence-base tools Fold recognition Model building Standalone programs Databases Model selection and ranking Hybrid methods combining template-based and The prediction of three-dimensional (3D) protein structure from amino acid sequences has stood as a significant challenge in computational and structural bioinformatics for decades. Recently, the widespread integration of artificial intelligence (AI) algorithms has substantially expedited advancements in protein structure prediction, yielding numerous The conformational parametersP k for each amino acid species (j=1–20) of sequential peptides in proteins are presented as the product ofP i,k, wherei is the number of the sequential residues in thekth conformational state (k=α-helix,Β-sheet,Β-turn, or unordered structure). Since the average parameter for ann-residue segment is related to the average
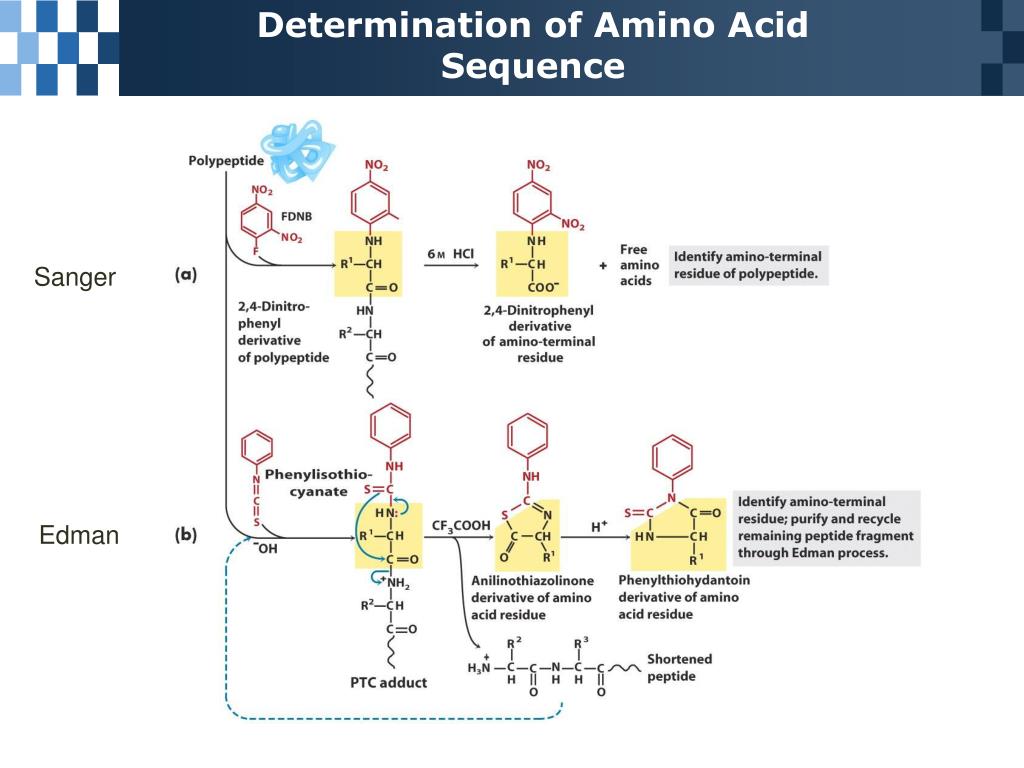
Prediction of amino acid sequence from structure.
Background The relationship between the sequence of a protein, its structure, and the resulting connection between its structure and function, is a foundational principle in biological science. Only recently has the computational prediction of protein structure based only on protein sequence been addressed effectively by AlphaFold, a neural network approach that can
Based on this relationship, we hypothesize that protein conformational dynamics are also determined, at least in part, by amino acid sequence and that this relationship may be leveraged for construction of AI/ML models dedicated to predicting ensembles of protein structures (i.e., distinct conformations).
The subunit structure and complete amino acid sequence of the lectin extracted from Lens culinaris (LcL) seeds was determined. In previous studies, the primary structure of the alpha-chain (Mr = 5,710) was shown to be homologous to the alpha-chain of the lectin from Pisum sativum, the Vicia cracca glucose-specific lectin, and a region in the middle of the concanavalin The amino acid sequence and protein structure have always been connected, and they need to be analyzed critically, which would enable the prediction of function from genome sequence data and facilitate the intentional modification of annotated protein functions by crafting amino acid sequences with targeted structures. SECONDARY STRUCTURE PREDICTION Protein secondary structure prediction refers to the prediction of the conformational state of each amino acid residue of a protein sequence as one of the three possible states, namely, helices, strands, or coils, denoted as H, E, and C, respectively.
In principle, it is possible to predict theoretically the three-dimensional structure of a protein from its amino acid sequence. Recently substantial progress towards this goal has been made by We have developed a method for the prediction of an amino acid sequence that is compatible with a three-dimensional backbone structure. Using only a backbone structure of a protein as input, the algorithm is capable of designing sequences that closely resemble natural members of the protein family to which the template structure belongs. In general, the Given the amino-acid sequence or 3D structure of a protein, how much can we predict about its function using just a desktop computer? The recent explosive growth in the volume of sequence data and
One of the key challenges in protein science is determining three dimensional structure from amino acid sequence. Although experimental methods for determining protein structures are providing high resolution structures, they cannot keep the pace at which amino acid sequences are resolved on the scale of entire genomes. We developed and evaluated a new predictor of protein function, functional annotator (FANN), from amino acid sequence. The predictor exploits a multioutput neural network framework which is well suited to simultaneously modeling
The document discusses various computational methods for predicting the three-dimensional structure of proteins from their amino acid sequences. It describes As we have seen previously, amino acids vary in their propensity to be found in alpha helices, beta strands, or reverse turns (beta bends, beta turns). These difference can be rationalized from the structure of each amino acid, as described before. Figure: Amino Acid Structure and propensity for secondary structure From the data bases, propensities can be calculated to
Sequence logos are a graphical representation of the information content stored in a multiple sequence alignment (MSA) and provide a compact and highly intuitive representation of the position-specific amino acid composition of binding motifs, active sites, etc. in biological sequences. (Reference: Thomsen, M.C., & Nielsen, M. 2012.
A tool that draws peptide primary structure and calculates theoretical peptide properties.
Since the gap between the number of the resolved protein structures and available protein sequences is continuously growing, it is important to provide computational tools for protein flexibility prediction from amino acid sequence. In this chapter, we explore how the tremendous progress in computational biology has transformed our approach to predicting protein structures from their amino
Residues distributions Performs a count of amino acid frequencies in peptide sequences. It is theoretically possible to predict the 3D structure of a protein just from its amino acid sequence. However, this is extremely challenging because of the sheer number of possible conformations. ProtParam [Documentation / Reference] is a tool which allows the computation of various physical and chemical parameters for a given protein stored in UniProtKB or for a user entered protein sequence. The computed parameters include the molecular weight, theoretical pI, amino acid composition, atomic composition, extinction coefficient, estimated half-life, instability index,
A tool for accurate prediction of a protein’s secondary structure from only its amino acid sequence with no evolutionary information i.e. MSA required. If there is no empirical model for your amino acid sequence, it may be useful to explore empirical models for closely-related sequences, if available. Even if an empirical structure is available, most have missing residues or atoms, and it may be useful to compare it with the AlphaFold prediction: see Missing residues and incomplete sidechains. Peptide Sequence Builder Peptide Sequence Builder is a straightforward yet powerful tool for constructing custom peptides by selecting from L- or D-amino acids and specifying the C-terminus. You can enter your sequence in either three-letter or one-letter code, and the tool instantly calculates the peptide’s molecular weight and formula.
Prediction of the Secondary Structure of Proteins from their Amino Acid Sequence Peter Y. Chou, Gerald D. Fasman Book Editor (s): Alton Meister Abstract This review attempts a critical stock-taking of the current state of the science aimed at predicting structural features of proteins from their amino acid sequences. At the primary structure level, methods are considered for detection of remotely related sequences and for recognizing amino acid patterns to predict posttranslational modifications and binding sites. The techniques
- Praxis Dr. Med. Matthias Schmidt
- Preemptive Scheduling Vs Non Preemptive Scheduling
- Preenchimento Gengival: Entenda Como Funciona Essa Técnica!
- Present German Plagen | afflict: English conjugation table
- Predicting Credit Card Delinquency
- Praxis Dr. Cathrin Sachse | Praxis Dr.med.dent. Cathrin Reuter
- Precios En Ibiza : [UNVRS] · Bienvenidos al Universo · 2025 · Compra tus entradas
- Praxis Für Oralchirurgie Radebeul
- Preisverleihung 2003 _ Bilder von Peter Abt aus der FU Physik
- Predicting Snatch And Clean , Snatch and Clean & Jerk: A Simplified Guide
- Preparing For Treatment With Good Nutrition
- Premier League: Berührt Mich Nicht
- Premium Wordpress | Managed hosting for WordPress
- Praxis-Check Für Unternehmen Der Versicherungsvermittlung