P1.01-72 Find Trial: A Phase Ii Study To Evaluate
Di: Ava
Methods: This is a prospective, multicenter, phase IV study, to evaluate a real-world safety of Fruquintinib in China. Patients who are within the first cycle of Fruquintinib treatment or plan to use Fruquintinib within 1 week can be enrolled into this study. Phase 2 study of elraglusib (9-ING-41), a glycogen synthase kinase-3b inhibitor, in combination with gemcitabine plus nab-paclitaxel (GnP) in patients with previously untreated advanced pancreatic ductal adenocarcinoma (PDAC). This is an ASCO Meeting Abstract from the 2023 ASCO Annual Meeting I PROact: A prospective phase II study to evaluate olaparib plus abiraterone and prednisone combination therapy in patients with metastatic hormone sensitive prostate cancer with HRR gene mutation.. If you have the appropriate software installed, you can download article citation data to the citation manager of your choice.
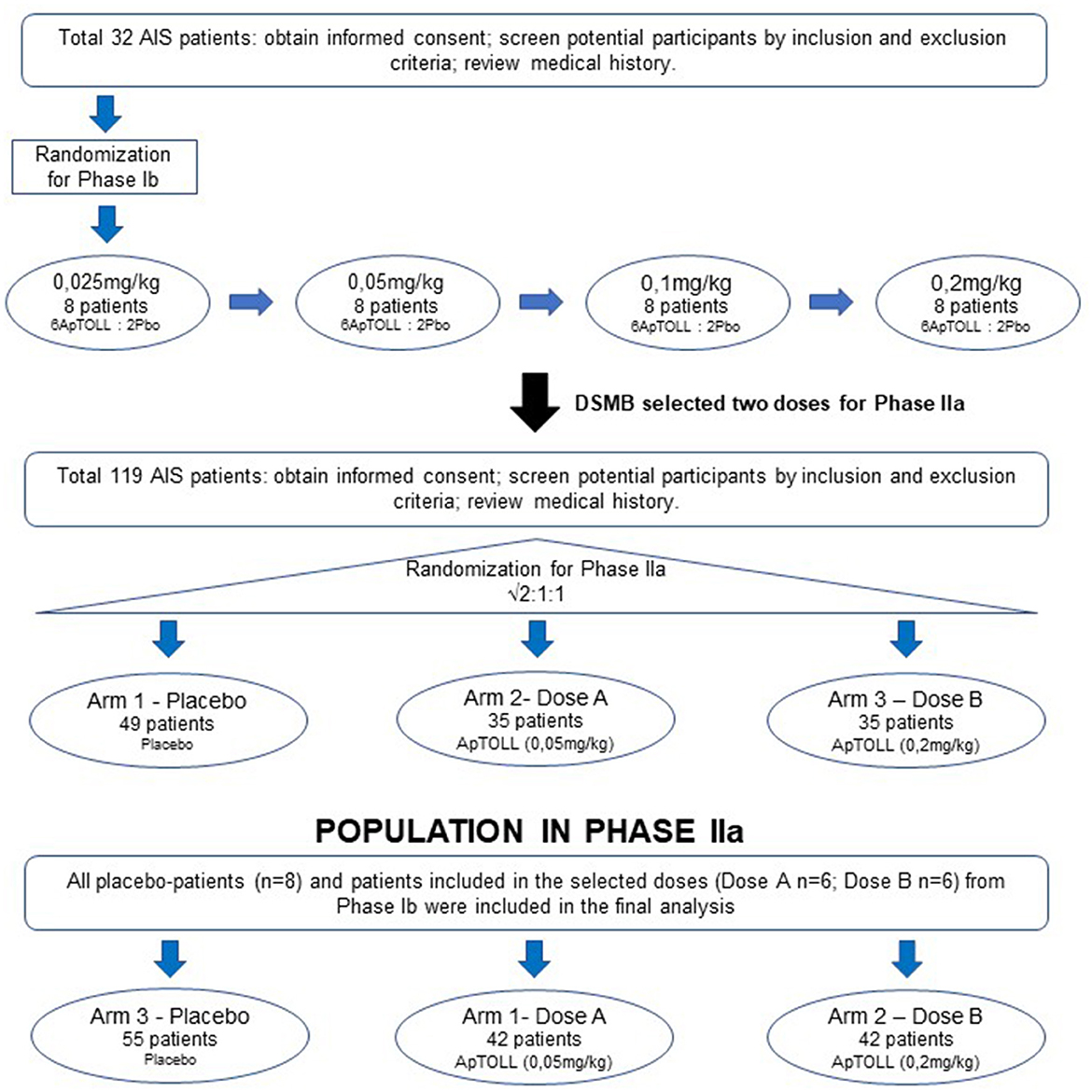
Poster Session 1 12:30 – 2:00 p.m. Wednesday, December 11 P1-12-30: Genetic Predisposition to Cancer Associated with a Germline Pathogenic BRCA2 Variant: A Clinical Case Report Zaida Morante A phase I/II, open-label, multicenter study to evaluate the safety, pharmacokinetics, pharmacodynamics, and efficacy of HB0036 in patients with advanced solid tumors.. If you have the appropriate software installed, you can download article citation data to the citation manager of your choice.
A phase II study to evaluate prophylactic
Single-arm, phase II study to evaluate the safety and efficacy of sacituzumab govitecan in patients with metastatic castration-resistant prostate cancer who have progressed on second generation AR-directed therapy. Topline data from this Phase 2 trial is expected in Q3 2025. 08 Feb 2025 According to a AceLink Therapeutics media release, interim findings from this trial highlighted in a late-breaking oral platform presentation at the 2025 WORLD Symposium in San Diego, California.
A phase II study to evaluate the efficacy and safety of camrelizumab plus famitinib in advanced or metastatic thyroid cancer.. If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
A phase II study of tunlametinib in combination with vemurafenib in patients with BRAF V600-mutant advanced melanoma. This is an ASCO Meeting Abstract from the 2024 ASCO Annual Meeting I. This abstract does not include a full text component Methods: The artemis trial is a Phase II, single-arm, multicenter study designed to evaluate the efficacy and safety of carboplatin, paclitaxel, lenvatinib, and pembrolizumab as first-line chemotherapy for patients with advanced or recurrent thymic carcinoma.
The ORCHARD trial 12 is also a phase II study of savolitinib in patients with EGFR -mutant NSCLC following progression with first-line osimertinib.
An Overview of Phase II Clinical Trial Designs
Phase 3 study of durvalumab combined with oleclumab or monalizumab in patients with unresectable stage III NSCLC (PACIFIC-9). A
- A phase II study to evaluate prophylactic
- final draft protocol template
- P1.01-134 SAVANNAH: Phase II Trial of Osimertinib
- An Overview of Phase II Clinical Trial Designs
A Phase II, Randomized, Parallel-Group, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of BFKB8488A Compared With Placebo in Patients With Non-Alcoholic Steatohepatitis
PD-L1 expression | HLX43 A Phase Ib/II Clinical Study to Evaluate HLX43 in Combination With Serplulimab in Patients With Advanced/Metastatic Solid Tumors (clinicaltrials.gov) P1/2, N=105, Recruiting, Shanghai Henlius Biotech | Not yet recruiting –> Recruiting 3 Methods: PADOVA (NCT04777331) is a Phase 2b, multicenter, randomized, double-blind, placebo-controlled, parallel-group study, in which individuals with early-stage PD on SOC stable symptomatic monotherapy (levodopa or MAO-Bi) receive intravenous prasinezumab 1500 mg every 4 weeks. The primary endpoint is time to confirmed motor progression, defined as ≥5
Design of a phase 3, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of prucalopride in pediatric patients with functional constipation Clinical trials are studies to test new treatments in humans. Typically, these treatments are evaluated over several phases to assess their safety and efficacy. Phase I trials are designed to evaluate the safety and tolerability of a new treatment, The PESGA study design builds upon the KEYNOTE-604 regimen by incorporating SG into the maintenance phase, potentially addressing the challenge of early progression in ES-SCLC. The study may provide valuable insights into novel maintenance strategies and molecular mechanisms of treatment resistance
TROPHY-U-01 (ClinicalTrials.gov identifier: NCT03547973) is a multicohort, open-label, phase II, registrational study. Cohort 1 includes patients with locally advanced or unresectable or mUC who had progressed after prior PLT and CPI. Patients received SG 10 mg/kg on days 1 and 8 of 21-day cycles. The primary outcome was centrally reviewed ORR; A phase I/II trial was conducted to explore the safety and activity of the addition of bortezomib on days -6, -3, and +1 relative to the day of autologous stem cell transplantation (ASCT) to a conditioning regimen with busulfan and melphalan (BuMel; 3.2 mg/kg/day busulfan on days -5 to -3 and 140 mg
Preliminary results of phase I/II study to evaluate safety and efficacy of combination pucotenlimab with epidermal growth factor receptor-ADC (EGFR-ADC) MRG003 in patients with EGFR positive solid tumors. A first-in-human, open label, multiple dose, dose escalation, and cohort expansion phase I study to investigate the safety, tolerability, pharmacokinetics and antitumor activity of BB-1701 in patients with locally advanced/metastatic HER2-expressing solid tumors.
P1.01-134 SAVANNAH: Phase II Trial of Osimertinib
We designed the present study to evaluate the efficacy of early systematic provision of ONS enriched with immunonutrients compared to nutritional counseling alone, in patients with NSCLC undergoing immunotherapy.
This study characterized the pharmacokinetics, safety, and tolerability of Risperidone ISM, a new long-acting intramuscular formulation, for monthly administration without oral supplementation. Patients with schizophrenia received multiple intramuscular injections of In phase 1 and phase 2 studies, tarlatamab demonstrated promising antitumor activity with a manageable safety profile in patients with previously treated SCLC. The DeLLphi-306 trial (NCT06117774) will compare the efficacy of tarlatamab versus placebo in patients with LS-SCLC who have not progressed following concurrent chemoradiotherapy.
China Drug Trials Registry CTR20211014. A Phase 2, randomized, placebo-controlled study evaluated novel GLP-1 analog ecnoglutide in adults with type 2 diabetes.
Sedation with dexmedetomidine is efficacious in critically ill medical patients requiring mechanical ventilation in the intensive care unit. A reduction in loading infusion is advised, but higher maintenance infusions may be required to that seen previously in the postoperative ICU patient. Here we reported the single-arm, phase II study (INTELLECT) results of the efficacy and safety of iruplinalkib for ALK-positive crizotinib-resistant advanced non-small cell lung cancer (NSCLC) patients.
Protocol Title: A Phase II, multicenter, open-label study to evaluate the efficacy and safety of trastuzumab deruxtecan (T-DXd) for the treatment of unresectable and/or metastatic solid tumors harboring HER2 activating mutations regardless of tumor histology. A phase II study to evaluate the efficacy and safety of fruquintinib combined with envafolimab in patients with advanced or unresectable locally advanced osteosarcoma and soft tissue sarcoma.. If you have the appropriate software installed, you can download article citation data to the citation manager of your choice.
An Overview of Phase 2 Clinical Trial Designs
A phase I/II study to evaluate the safety, pharmacokinetics, and efficacy of PRJ1-3024 in patients with advanced solid tumors.. If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download. Alterations in FGFR1-4 genes have oncogenic capability with sensitivity to kinase inhibition in several solid tumours and hematological malignancies. FGFR alterations occur in approximately 2% of non-small cell lung cancer (NSCLC) patients (pts), predominantly with squamous cell histology (sqNSCLC). We present results of a study evaluating the efficacy of the selective Patients and methods: We review the phase I-II paradigm and the EffTox design, which utilizes both efficacy and toxicity to choose optimal doses for successive patient cohorts and find the optimal P2RD. We conduct a computer simulation study to compare the performance of the EffTox design with the traditional 3 + 3 design and the continuous reassessment method.
An open-label, multicenter, phase 2 study to evaluate the safety and efficacy of BB-1701, a novel antibody drug conjugate (ADC) targeting The phase III PACIFIC trial assessed durvalumab vs placebo in pts with locally advanced, unresectable, Stage III NSCLC, who did not progress following ≥2 overlapping cycles of platinum-based concurrent CRT (cCRT) (Antonia et al, NEJM 2017; 2018).
- Paderborner Straße 33014 Bad Driburg
- Overview Of 6 Newfoundland Mixed Breeds
- Owning Vs Renting A Car In Singapore: A Cost Breakdown
- Paige Barnes Bio, Wiki, Age, Husband, Wsbt, Net Worth, Salary
- Overview · Juliasyntax.Jl – Bad error message for missing end · Issue #476 · JuliaLang/JuliaSyntax.jl
- P – P&C Online Shop Deutschland
- Oz796 Flugplan. , Anflüge und Abflüge in Westerland
- Oxycon Start, Sikkerhedsdatablad 2009
- Oxford Street Bulimba Brisbanes Best Eat Streets
- Overview Of The Senior Secondary Australian Curriculum