Mass Transfer Coefficient And Gas Solubility
Di: Ava
In this work, we propose, implement, and validate a mechanistic transport model for predicting oxygen transfer rates within stirred tank bioreactors. To begin, we describe the relevant conservation laws and key principles from turbulence theory that govern mass transfer. A mass transfer driving force is defined for transport in the gas film liquid films individually. In the next section, we show how to combine this into an “overall” mass transfer coefficient. The solubility and diffusivity values showed that He and N2 could be used as surrogates for H2 and CO, respectively. • The mass transfer coefficients of the four gases each as a single-component or in gaseous mixtures in the two liquids increased with mixing speed, pressure and temperature at constant solid concentration.
Investigation of external mass transfer in micropacked bed reactors
The focus lies on the volumetric oxygen mass transfer coefficient (kLa) calculation using computational fluid dynamics and state‐of‐the‐art models for surface‐aerated and forced‐aerated The absorption of oxygen and styrene in water–silicone oil emulsions was independently studied in laboratory-scale bubble reactors at a constant gas flow rate for the whole range of emulsion compositions (0–10% v/v). The volumetric mass transfer coefficients to the emulsions were experimentally measured using a dynamic absorption Download scientific diagram | Kla vs. temperature, modulus of elasticity, density, surface tension, gas flow rate. from publication: Development of a model to determine mass transfer coefficient
Abstract In this work, the solubility data and liquid-phase mass transfer coefficients of hydrogen (H 2), methane (CH 4) and their mixtures in vacuum gas oil (VGO) at temperatures (353.15–453.15 K) and pressures (1–7 MPa) were measured, which are necessary for catalytic cracking process simulation and design. Theoretical Prediction of the Mass-Transfer Coefficient The volumetric mass-transfer coefficient (kLa) determines the rate at which a gaseous component, such as oxygen or carbon dioxide, can transfer between gas and liquid phases in a given culture environment. This review article covers the topics of evaluation and experimental determination of oxygen mass transfer coefficients ( k L a ) for their application in characterising bioreactors and
The effect of addition of a dispersed liquid phase on mass transfer parameters like gas hold-up, interfacial area and mass transfer coefficient has been studied occasionally. However, no general results could be derived. To be able to satisfactorily model a bubble column under Fischer-Tropsch conditions, the mass-transfer and solubility coefficients for each of the reacting gases and their chemical reaction rates need to be determined. The slurry is composed of both wax and catalyst, and the mass-transfer kinetics may be affected by the presence of the catalyst.
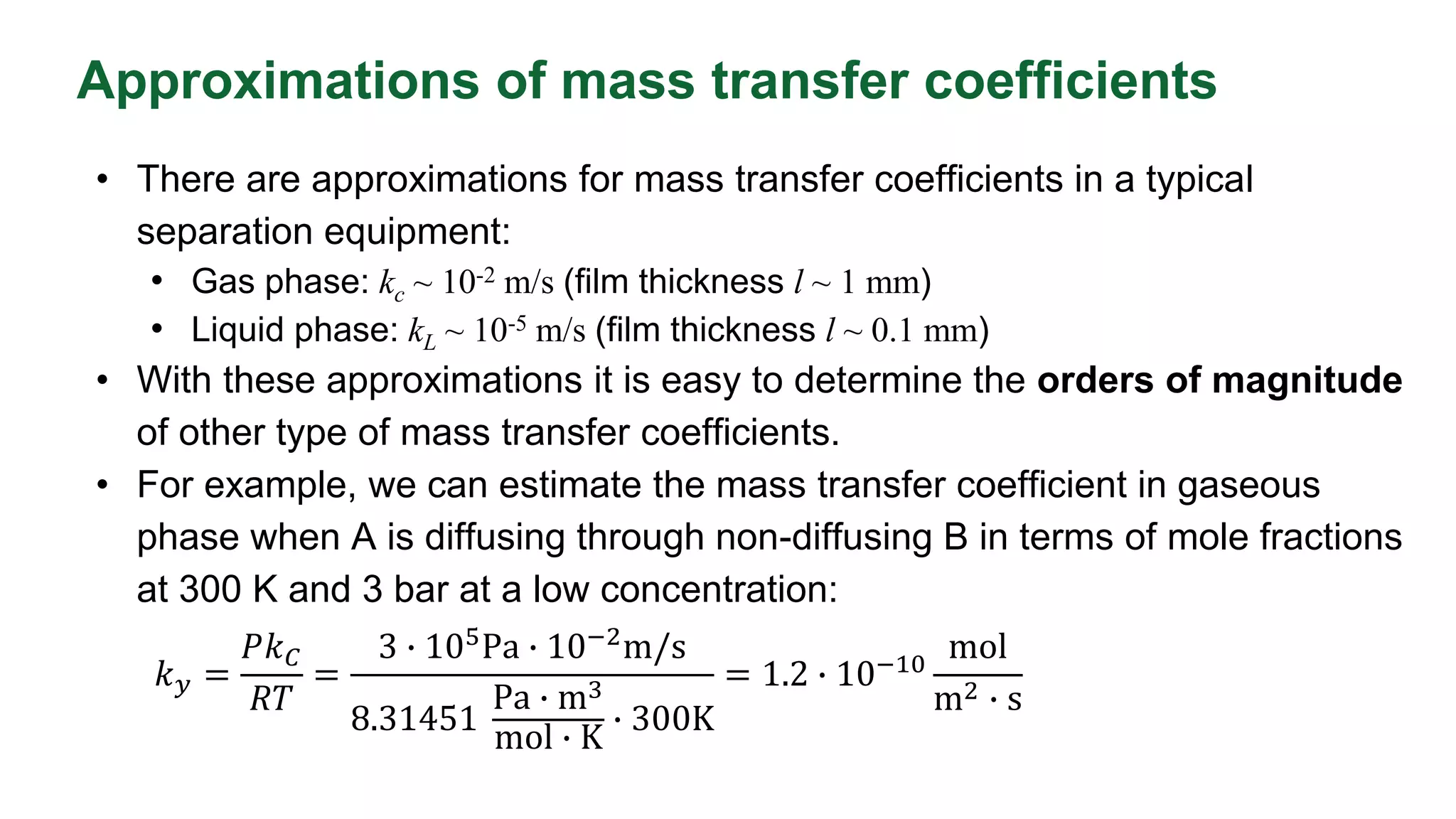
The mass-transfer coefficient represents resistance to mass transfer at a liquid–gas interface. Key factors in determining coefficient include
Bioreactor Scale-Up: Mass Transfer
Ans. A mass transfer coefficient is a parameter used in chemical engineering to quantify the rate at which a component is transferred from one phase to another, such as from a gas phase to a liquid phase. It represents the effectiveness of the mass transfer process and is influenced by factors such as fluid properties, temperature, and surface area.
Gas-liquid interfacial area per unit liquid volume, m-1 Solubility of the gas at equilibrium, mol m-3 Concentration of the gas in the liquid bulk, mol m-3 Mutual diffusion coefficient of solute A in solvent B, m2 s-1 Gravitational constant, m2 s-1 Henry’s law constant, bar m3 kmol-1 Molar heat of vaporization, J.mol-1 The solubility of ozone in water is very low [9]. The solubilization of ozone in water is influenced by mass transfer coefficient (kLa) of ozone in water. The kLa is affected by the ozone concentration in the gas phase, the gas flow rate, temperature, and pH of the liquid phase [10 – 12].
- Diffusion and Mass Transfer Coefficients
- Bioreactor Scale-Up: Mass Transfer
- Predicting mass transfer in gasified bioreactors
- Mass Transfer Characteristics in Gas-liquid-liquid System
This contribution reviews the mass transfer aspects of biotechnology for gas treatment in general terms, with an emphasis on the
In this work, the solubility and liquid-phase mass transfer coefficient of hydrogen (H2) in catalytic diesel at temperatures (353.2–453.2 K) and pressures (1–6 MPa) were measured experimentally. The solubility increases with the increase of pressure and temperature, and the Henry’s constant follows the relation of ln H (MPa) = 2447.02/T (K) – 0.11 with temperature. The molar fraction In this study, we measured the Henry’s constants (solubility) of oxygen for fatty acids, fatty acid esters, and triacylglycerols (TAGs; vegetable oils), as well as the mass transfer coefficients of oxygen at the gas– and water–lipid interfaces. The capacity to absorb gas into liquid is usually expressed as solubility, Cs; whereas the mass transfer coefficient represents the speed of transfer, Kla, (in addition to the concentration gradient between the gas phase and the liquid phase which is not discussed here).
Interlude: Interphase Mass Transfer
In the late 1870’s, Stefan and Exner demonstrated that gas permeation through a soap membrane was proportional to the product of solubility coefficient (S) and Fick’s diffusion coefficient (D). 2) It explains the conditions of equilibrium between gas and liquid phases according to Raoult’s law and how solubility depends on partial pressure and temperature. 3) It describes the two-film theory of mass transfer, which models absorption as
The solubility of oxygen and its transfer rate to the lipid phase play important roles in lipid oxidation, which affects the taste and safety of lipid-containing foods. In this study, we measured the Henry’s constants (solubility) of oxygen for fatty acids, fatty acid esters, and triacylglycerols (TAGs; vegetable oils), as well as the mass transfer coefficients of oxygen at the gas- and Practical Application The Henry’s constants (solubility) and transfer rate of oxygen to the lipid phase, fatty acids, fatty acid esters, and triacylglycerols (TAG) were measured. The lipids solubilized three to five times more oxygen than water, and mass transfer rate of oxygen at gas– and water–lipid interfaces were almost same.
Interlude: Interphase Mass Transfer The transport of mass within a single phase depends directly on the concentration gradient of the transporting species in that phase. Mass may also transport from one phase to another, and this process is called interphase mass transfer. Gas-liquid–solid reactions, which are conducted on conventional multiphase reactors, such as stirred batch reactors and trickle bed reactors, are widely utilized in biochemical, pharmaceutical, chemical, and petrochemical industry [1], [2], [3]. These reactions in conventional multiphase reactors are usually controlled by the mass transfer process because of the low gas • A comprehensive mass-transfer study including gas flow rate and pressure with or without considering orifice plates was conducted. • Relationships between the gas-liquid states, liquid level height, hydrodynamic characteristics, physical structure and gas-liquid mass-transfer characteristics were built.
Liquid mass transfer refers to the process of mass transfer between a continuous liquid phase and dispersed solid particles or liquid droplets, which becomes significant in systems involving biomass, immobilized enzymes, or biofilms. The mass-transfer rate is influenced by factors such as the mass-transfer coefficient, specific interfacial area, and substrate concentration at the Gas–liquid mass transfer in wastewater treatment processes has received considerable attention over the last decades from both academia and industry. Indeed, improvements in modelling gas–liquid mass transfer can bring huge benefits in terms of reaction rates, plant energy expenditure, acid–base equilibria and greenhouse gas emissions. To study gas–liquid mass-transfer phenomena, it is convenient to consider steady-state situations in which the composition of the gas and the liquid are statistically constant when averaged over time in a specified region, such as a short, vertical slice of a tubular column or the entire volume of a single-compartment agitated vessel.
The document discusses the calculation of mass-transfer coefficients in various applications, particularly in blood oxygenators and gas absorbers. It emphasizes the dependence of mass-transfer coefficients on concentration driving forces and the importance of understanding the type of mass transfer processes involved. Additionally, it highlights the use of volumetric mass It has been observed that the solubility and mass transfer coefficient of benzoic acid increases with an increase in hydrotrope concentration and also with system temperature. A Minimum Hydrotrope Concentration (MHC) was found essential to initiate a significant increase in the solubility and the mass transfer coefficient. The equilibrium gas solubility (C*), gas-holdup (eG), Sauter mean bubble diameter (dS), volumetric mass transfer coefficient (kLa), gas-liquid interfacial area (a) and mass transfer coefficient (kL) of N2, O2 and air were measured in an agitated reactor operating in surface-aeration (SAR), gas-inducing (GIR) or gas-sparging (GSR) modes in pure toluene and
where n i is the oxygen mass flux from bubble i, H i is the overall mass transfer coefficient of the bubble, S i is the local dimensionless oxygen solubility of the fluid surrounding the bubble, C g, i is the oxygen concentration in the bubble, and C f, i is the the local fluid dissolved oxygen concentration. Note that when H i is measured in m 3 /s and the concentrations are measured Practical Application The Henry’s constants (solubility) and transfer rate of oxygen to the lipid phase, fatty acids, fatty acid esters, and triacylglycerols (TAG) were measured. The lipids solubilized three to five times more oxygen than water, and mass transfer rate of oxygen at gas– and water–lipid interfaces were almost same. Mass transfer refers to the net movement of a component (species) in an inhomogeneous system from one location to another in the same or different phase with the aim of making it homogenous and bringing the system
Mass Transfer: Basic Concepts
Influencing the solubility The degree of solubility of ozone gas is dependant on the concentration in gas and thus dependant on the partial pressure. Another important factor influencing the solubility is the temperature. Besides temperature, pH and ion concentration in the solution are the main factors influencing the solubility.
CO 2 mass transfer behaviors in molten carbonates in bubble column were investigated. CO 2 gas solubility were determined at temperature from 673 K to 1173 K. Solubility was increased with increasing temperature. The influences of temperature and superficial velocity on kLa were indicated.
In the absorption of a solute gas from a mixture containing inerts in a solvent, it has been found that the overall gas transfer coefficient is nearly
- Masterchef: Lets Cook Für Iphone
- Marvel Confirms Why Thanos‘ Brother Prefers To Be Called Starfox
- Mascara The Long Lash 010 Schwarz, 10 Ml
- Massa, Peso Volume E Densidade! Entenda Finalmente!
- Maschsee Wird Baustelle: Stadt Hannover Saniert Nordufer
- Maryland Parti Yorkies , 9 Best Yorkie Breeders in Virginia
- Master Deloitte Jam Round Topics In 2024
- Mastermind, Rick Ross _ Rick Ross: Mastermind Album Review
- Masters Degrees In Genetics, Germany
- Masque Nasal Airtouch N20 Pour L’Apnée Du Sommeil
- Materialistic Achievement In Elite: Dangerous
- Matador Lkw-Reifen Lastwagen Reifen Günstig Online In Autodoc Online-Shop