Magnesium Sulphate : Structure, Properties And Uses
Di: Ava
Yet, the crystal of magnesium chloride can take the structure of zinc-blende and wurtzite structure by the process of molecular beam epitaxy. The chemical properties of this compound, MgS the ? Discover the essentials of Potassium Sulfate (K₂SO₄): its definition, structure, preparation methods, key properties, versatile uses in agriculture and industry and its side Chemical Properties Magnesium sulfate is a white crystalline solid and odourless chemical compounds. It is insoluble in the acetone and it is soluble in alcohol and glycerol. It is
Potassium sulfate (US) or potassium sulphate (UK), also called sulphate of potash (SOP), arcanite, or archaically potash of sulfur, is the inorganic compound with formula K 2 SO 4, a
Magnesium Sulfate Heptahydrate
Magnesium sulphide nanoparticles show potential for use as biomedical imaging agents and drug carriers due to their chemical stability, low toxicity and optical properties. Magnesium sulphide magnesium sulfate Properties Magnesium solfate is a mineral compound whose chemical composition consists of magnesium, sulfur and oxygen. Its chemical formula is MgSO4.
Magnesium sulfate or magnesium sulphate is a chemical compound, a salt with the formula MgSO₄, consisting of magnesium cations Mg²⁺ and sulfate anions SO2−4. Concerning the above mentioned magnesium ion role and the need of materials of beneficial properties the magnesium sulphate complex compounds with hmta and phen were
Magnesium sulfate is sometimes used as a mordant for dyes. Magnesium hydroxide is added to plastics to make them fire retardant. Magnesium oxide is used to make heat-resistant bricks for Master vitriol’s structure, properties, and uses with expert tips. Boost your Chemistry skills with Vedantu-learn more now!
- The role and significance of Magnesium in modern day
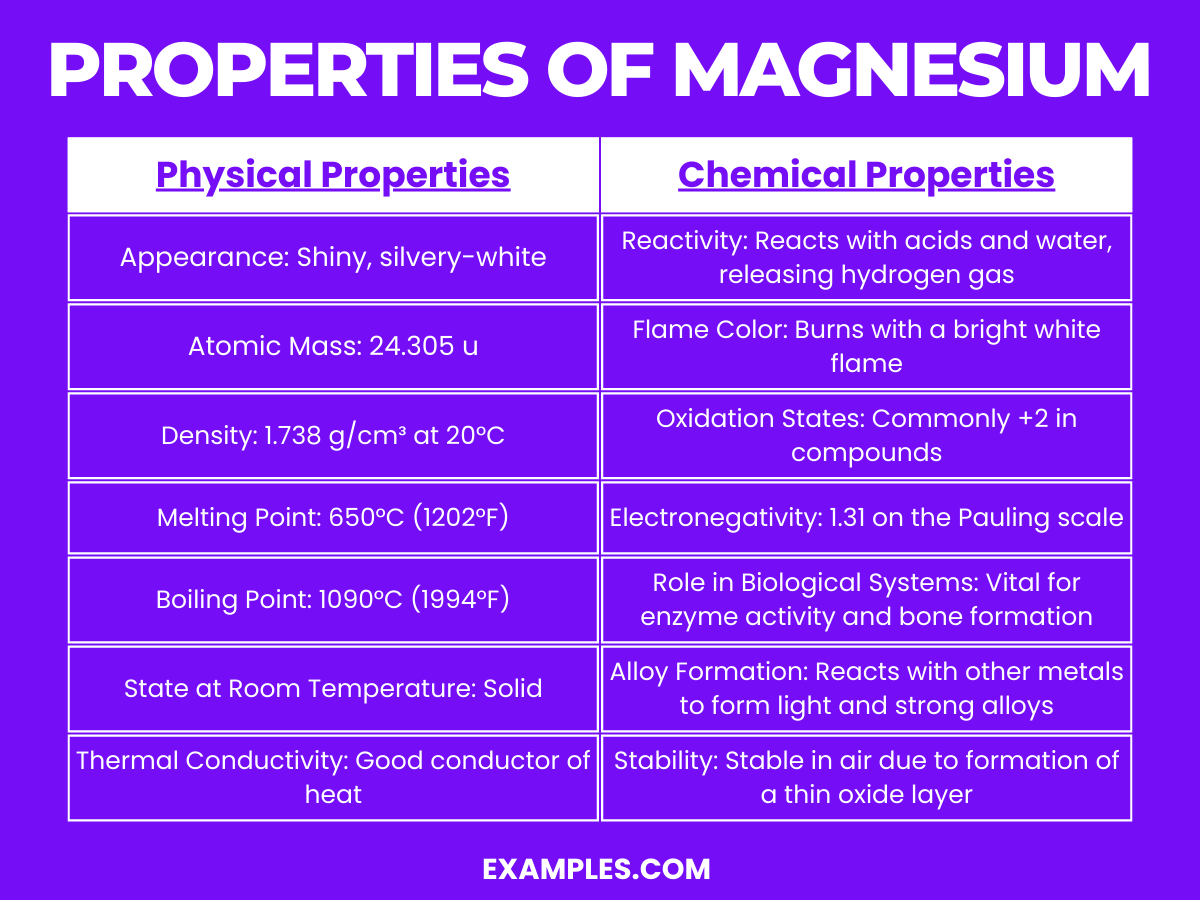
- Magnesium Element: Properties, Uses, Interesting Facts
- Magnesium Sulfate Heptahydrate
Formula and structure: The chemical formula of magnesium sulfate is MgSO 4 and its molar mass is 120.366 g/mol. It also commonly exists as the monohydrate form (with one molecule of
Sulphate is a chemical compound made up of sulphur atoms and oxygen atoms. Potassium, sodium, calcium, magnesium, and barium are some of the elements that form salts with sulphate. 1. Summary Magnesium sulfate was evaluated by the Committee at its 63rd meeting. The outstanding information on functional uses other than nutrient supplement as well as Magnesium sulfate is used in chemistry and molecular biology as a source of magnesium ions. Magnesium has a variety of biological roles in enzymology, cell membrane and wall structural
Epsom salt is also known as magnesium sulfate and bitter salt. There are three different forms, a heptahydrate, anhydrous and monohydrate form. This chemical compound Impurities in the form of calcium chloride, sodium sulfate, and magnesium chloride are present in the sodium obtained from the crystallization of brine. The coarse salt is dissolved Some of the sulfate uses are listed below. Magnesium sulfate is used in therapeutic baths. Sulphate minerals are used in the making of metal salts. Copper sulfate is the most common
Runčevski used citric acid as a modifier to analyze the molecular structure of 5-1-7 whiskers, the main hydration product of BMSC. In terms of mechanical properties, setting time, Sulfate and sulfonate are both chemical compounds that contain sulfur, but they differ in their chemical structures and properties. Sulfate is an anion with the formula SO4^2-, consisting of a MAGNESIUM LAURETH SULFATE (CAS 62755-21-9) information, including chemical properties, structure, melting point, boiling point, density, formula, molecular weight,
From improving soil structure to enhancing crop yields, magnesium sulphate fertilizer plays a vital role in modern agriculture. Epsom salt has been used as a natural remedy for hundreds of years. Learn more about its uses, benefits, and side effects.
Magnesium sulfate is a drug used to treat convulsions during pregnancy, nephritis in children, magnesium deficiency, and tetany.
Magnesium sulfate is an important raw material for MOS and BMSC. To reduce the cost of reactive magnesite-based cementitious materials, industrial-grade magnesium sulfate The optical properties were studied by using UV-Vis analysis; all crystals have shown good absorption in the infrared and visible area of the spectrum. The characteristic
Chemical Structure Sulfates are chemical compounds that contain the sulfate ion (SO4^2-), which consists of a central sulfur atom bonded to four oxygen atoms. Sulfates are commonly found in
Magnesium sulfate heptahydrate (CAS 10034-99-8) information, including chemical properties, structure, melting point, boiling point, density, formula, molecular weight, Occurrence of Magnesium Magnesium is the eighth most prevalent element in the Earth’s crust (approximately 2.5 percent) and the third most abundant structural metal after
Dive deeper into the world of magnesium and find out more about what it is, its properties, structure, and uses.
Formula of Sulphate ion: Sulphate ion is basically made up of Sulphur and oxygen atoms. Sulphate ion is present in sulphuric acid which is called the king of chemicals since it is
Master magnesium nitrate-formula, structure, uses with easy notes. Boost your chemistry skills with Vedantu’s expert tips. Magnesium sulfate is sometimes used as a mordant for dyes. Magnesium hydroxide is added to plastics to make them fire retardant. Magnesium Magnesium sulphate is a widely used chemical that is available in various forms, depending on the amount of water in the crystal structure. Two of the most commonly found
Agricultural Applications of Magnesium Sulfate Salt Magnesium sulfate salt is widely used in agriculture as a soil amendment that improves plant nutrition. It contributes to
Sulphate Sulphate, also spelt sulfate, is a chemical compound that contains the sulfate ion (SO4)2−. The sulfate ion consists of one sulfur atom surrounded by four oxygen atoms in a Learn about Sulphate (SO₄²⁻) its definition, structure, properties, preparation, common examples, industrial uses, and potential hazards. ⇒ Sulphate Ion Formula: SO 42- Sulphate is basically a chemical compound that is composed of sulphur and oxygen atoms. Sulphate forms salts with
Magnesium Sulfate Heptahydrate | H14MgO11S | CID 24843 – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities,
- Maillot — Wikipédia – François Maillot — Wikipédia
- Maersk Jaipur, Container Ship , AS CALIFORNIA, Container Ship
- Mainz Dom Wandteller Zierteller Kupferteller Deko • Eur 5,00
- Maintenir L’Alimentation Des Ports Usb Du Pc Même Éteint
- Mail Und Fotos In Originalgröße
- Maglite Taschenlampe Ml150Lrx Led, 1082 Lumen, Mit Akku
- Maisonette Kaufen In Tutzing _ Loft kaufen in Tutzing, Tutzing
- Magnum P.I. Season 5 Episode 19
- Madeleine Definition In American English
- Magnetsystem Schwarz, Mit Gewindebuchse M4, Ø 43 Mm
- Maersk Führt Auflieger-Flotte An
- Mafia Patch 1.2 Problem | Mafia: The City of Lost Heaven GAME PATCH v.1.2 ENG