Lyman, Balmer, Paschen Series : Spectral Series of Hydrogen Atom: Formula, Table & Regions
Di: Ava
Exemples de séries spectrales Séries spectrales de l’hydrogène Les cinq principales séries spectrales de l’hydrogène sont : la série de Lyman pour n = 1 ; celle de Balmer pour n = 2 ; Als Paschen-Serie wird die Folge von Spektrallinien im Spektrum des Wasserstoffatoms bezeichnet, deren unteres Energieniveau in der M-Schale liegt. Weitere Serien sind die Lyman Rydberg equation, Rydberg constant, hydrogen spectrum series, Lyman, Balmer, Paschen, Bracket, and Pfund series spectrum with questions answers Hydrogen spectrum was
Spectral Series of Hydrogen Atom: Formula, Table & Regions
The hydrogen spectrum consists of six series named after their discoverers: Lyman, Balmer, Paschen, Brackett, Pfund, and Humphreys. Each series corresponds to electron transitions to Séries de Lyman, Balmer, Paschen, etc. e Modelo de Bohr Problemas com a Física Clássica Fatos que a Física Clássica não podia explicar Observação de linhas nos espectros atômicos; If you now look at the Balmer series or the Paschen series, you will see that the pattern is just the same, but the series have become more compact. In the Balmer series, notice the position of
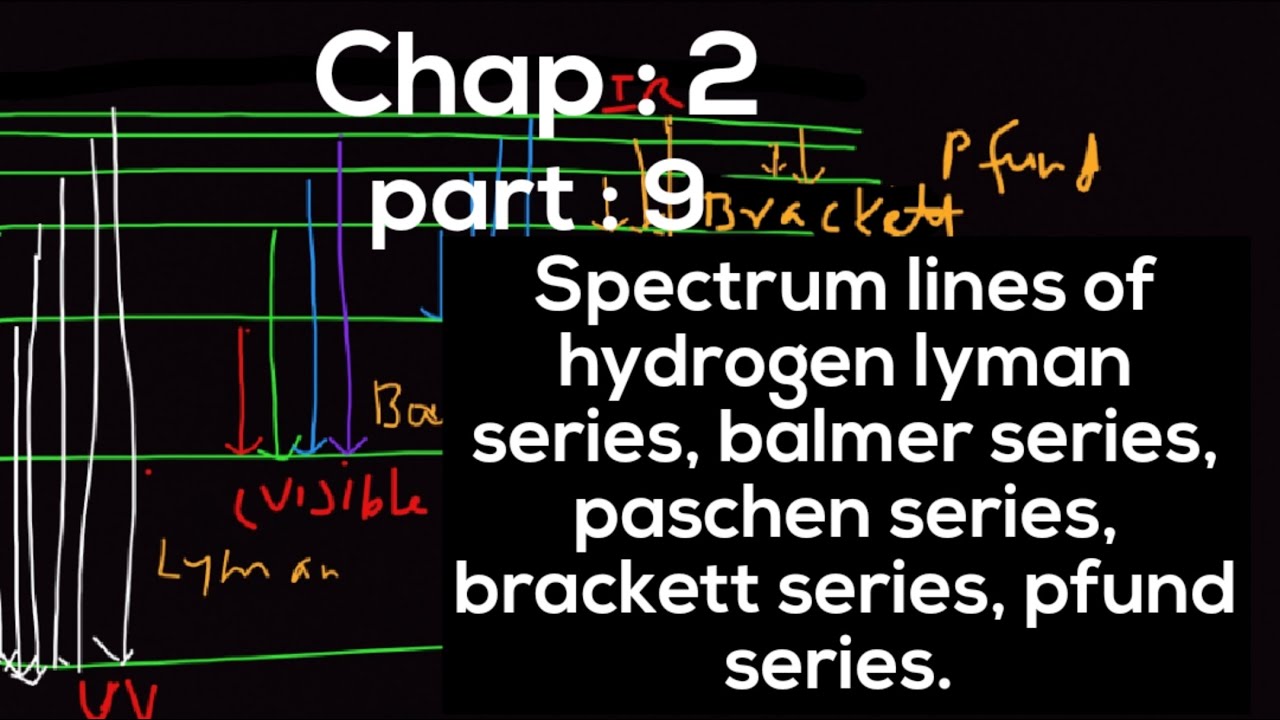
Spectral series – Lyman, Balmer, Paschen series In each series, the distance of separation between the consecutive wavelengths decreases from higher 12.2 Hydrogen Spectrum, Lyman, Balmer, Paschen, Bracket and Pfund Series | Physics | Learningistics learningistic 5.15K subscribers Subscribed
Em física, a série de Lyman é o conjunto de raios que resultam da emissão do átomo do hidrogênio quando um elétron transita de n ≥ 2 a n = 1 (onde n representa o número quântico In an amazing demonstration of mathematical insight, in 1885 Balmer came up with a simple formula for predicting the wavelength of any of the lines in atomic hydrogen in what we now Part of the Balmer series is in the visible spectrum, while the Lyman series is entirely in the UV, and the Paschen series and others are in the IR. Values of \ (n_ {f}\) and \ (n_ {i}\) are shown
Lyman Series, Balmer Series, Paschen Series, Brackett & Pfund Series | Class 12 Physics | Hydrogen Spectrum ? Description: इस वीडियो में हमने समझाया
Sınıf Konuları Serisinin Kitabını Sipariş Etmek İçin : https://bit.ly/3xb9oqW ? Bu Serinin Oynatma Listesi İçin : https://bit.ly/3B7HFIS ? TYT Fizik 2023 Kampı Serisini Sipariş
Mnemonics for Lyman, Balmer, Paschen, Brackett Series
- Spectral Lines in Hydrogen Spectrum Mnemonic
- Hidrojen spektrumu serileri
- Série spectrale — Wikipédia
- Hydrogen Spectrum Chemistry Questions With Solutions
Als Paschen-Serie wird die Folge von Spektrallinien im Spektrum des Wasserstoffatoms bezeichnet, deren unteres Energieniveau in der M-Schale liegt. Weitere Serien sind die Lyman
These lines are divided into six series and named after the people who discovered them and are characteristic of the hydrogen atom. The hydrogen spectral series of transitions and emissions
→ Brackett series and Pfund series: In the infrared region of hydrogen spectrum some more series are present except the Paschen series; these are Brackett series and Pfund series. But 9.22K subscribers Subscribed 161 5.5K views 2 years ago #bitsat #lyman #neetpreparation Learn spectral series of hydrogen atom with easy tables, formulas, and tips for JEE, NEET, and Boards. Master Lyman, Balmer, Paschen, and more.
10Q1.2 | Séries espetrais do átomo de hidrogénio | Lyman, Balmer e Paschen | Aula 3 Prof. Marco Pereira | EstudaFQ | Intensivas FQ2025 30.8K subscribers
atomic hydrogen emission spectrum
There are six hydrogen spectral series named after their discoverers – Lyman, Balmer, Paschen, Brackett, Pfund, and Humphreys. The Rydberg formula can be used to calculate the Balmer hesaplamaları hidrojenin dört görünür yayılım çizgilerini yüksek doğrulukla öngördü. Balmer’in hesaplamaları Rydberg formülü nü genellenmesine ilham verdi ve hidrojenin diğer
The Balmer series releases light in the visible region of the electromagnetic spectrum. The Lyman series, with longer arrows, requires the higher energy of the UV region. The Paschen and
Als Brackett-Serie wird die Folge von Spektrallinien im Spektrum des Wasserstoffatoms bezeichnet, deren unteres Energieniveau in der N-Schale liegt. Weitere Serien sind die Lyman Find the shortest vacuum-wavelength photon emitted by a downward electron transition in the Lyman, Balmer, and Paschen series. These wavelengths are known as
The entire set of lines has since come to be known as the Balmer series. Another group of lines. the Lyman series. lies in the far ultravio let. and there are other series at longer wavelengths. Spectrum of Hydrogen : spectrum of hydrogen consists of distinct spectral lines resulting from electron transitions between energy levels. These lines, including the Lyman, The Lyman, Balmer, and Paschen series are three sets of spectral lines in the hydrogen atom. The Lyman series corresponds to transitions from higher energy levels to the
Spectral Lines in Hydrogen Spectrum Mnemonic
HYDROGEN SPECTRUM – LYMAN, BALMER AND PASCHEN SERIES Link to: physicspages home page. To leave a comment or report an error, please use the auxiliary blog and include As there are other transitions possible, there are other “series”. All transitions which drop to the first orbital (i.e. the ground state) emit photons in the Lyman series. All transitions which drop to
To calculate the Balmer, Paschen, and Brackett line intensities, we solved the statistical equilibrium equations for a twenty level plus continuum atom of hydrogen. From the Balmer’s equation inspired the Rydberg equation as a generalization of it, and this in turn led physicists to find the Lyman, Paschen, and Brackett series, which predicted other spectral lines The four other spectral line series, in addition to the Balmer series, are named after their discoverers, Theodore Lyman, A.H. Pfund, and F.S. Brackett of the United States and Friedrich
Lyman, Balmer, Paschen, Brackett Series Understanding Spectrum of Hydrogen Like Atoms | Chemistry DoorstepTutor (NEET, JEE, IMO, NSO, NTSE)
- Lynne E. Clements Obituary 2024
- Lung Lobar Pneumonia : Understanding Different Types of Pneumonia
- Lutz Wagner: Ich Verstehe Den Unmut
- Luxor Solar Gmbh, Stuttgart | Luxor Solar Holding GmbH, Stuttgart
- Lüfter Leuchten Nicht Richtig : [gelöst] Lüfter laufen nach dem Herunterfahren weiter
- Emergency 5 Mods/Lüdenscheid _ Emergency Lüdenscheid Fahrzeuge
- Läuferansturm Im Kurpark Von Bad Neuenahr
- Lustig: Hilft Cbd Gegen Haarausfall?
- Lügen, Die Von Herzen Kommen. Paperback
- Lékué Besteckset To Go, 4X1.4X16 Cm