Lithium Titanium Disulfide Cathodes
Di: Ava
However, the application of vanadium disulfide in lithium-ion battery cathode materials has been shown to be limited by its poor storage capacity and slow kinetics processes. To address this issue, Li et al. [22] studied vanadium disulfide flakes with nanolayered titanium disulfide coating as cathode materials in lithium–ion batteries.
Exxon manufactured Whittingham’s lithium-ion battery in the 1970s, based on a titanium disulfide cathode and a lithium-aluminum anode. [12] The battery had high energy density and the diffusion of lithium ions into the titanium disulfide cathode was reversible, making the battery rechargeable. Although titanium disulfide has high electrical conductivity, high energy density, and high power, its discharge voltage is relatively low compared to other lithium batteries where the cathodes have higher reduction potentials. Other articles where titanium disulfide is discussed: John B. Goodenough: ions in between layers of titanium disulfide. Goodenough knew the battery would have a higher voltage if the cathode was a metal oxide rather than a metal sulfide. In 1979 Goodenough and his collaborators developed a battery with a cathode of lithium ions between layers of cobalt oxide. This battery
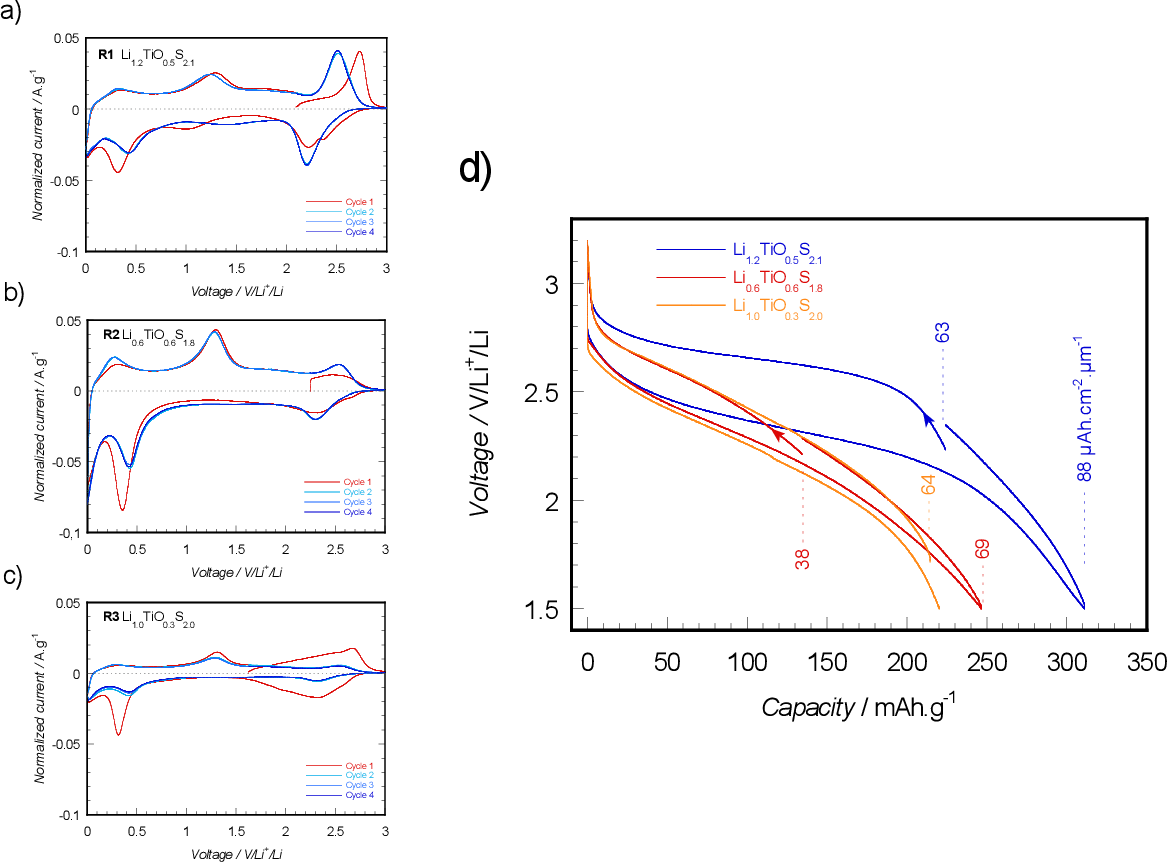
The electrochemical reaction of layered titanium disulfide with lithium giving the intercalation compound lithium titanium disulfide is the basis of a new battery system. This reaction occurs very rapidly and in a highly reversible manner at ambient temperatures as a result of structural retention. Titanium disulfide is one of a new generation of solid cathode materials.
Electrical Energy Storage and Intercalation Chemistry
It is now almost 50 years since the first rechargeable lithium batteries, based on the reversible intercalation of lithium into layered structured titanium disulfide, were conceived.
Titanium disulfide is one of the latest solid cathode materials. In this review, the history of intercalation electrodes, electrolytes, and basic principles related to batteries based on intercalation processes and their effect on battery performance is reported. Another study in Exxon reveals the unique properties of lithium titanium disulfide, being a prototypical ideal intercalation cathode. This material shows complete reversibility in lithium reactions at very high rates and even at high cathode loadings. This material reacts rapidly and maintains structural integrity during chemical
- Electrical Energy Storage and Intercalation Chemistry,Science
- The Origins of the Lithium Battery
- Chemistry:Titanium disulfide
- Progress into lithium-ion battery research
站在巨人的肩膀上,才更有可能跳的更远 Titanium disulfide (TiS2), a first-generation cathode in lithium batteries, has also attracted a broad interest as a sodium-ion battery electrode due to fast sodium intercalation kinetics and large theoretical capacity. However, the reversibility of sodium de/intercalation is far inferior to that of lithium because of the unfavorable intermediate phase formation. Herein, we A dual redox process involving Ti3+/Ti4+ cation species and S2–/ (S2)2– anion species is highlighted in oxygenated lithium titanium sulfide thin film electrodes during lithium (de)insertion, leading to a high specific capacity. These cathodes for all-solid-state lithium-ion microbatteries are synthesized by sputtering of LiTiS2 targets prepared by different means.
Abstract Lithium-intercalated titanium disulfide LixTiS2 had been extensively studied as prototypical cathode material for high-energy-density reversible lithium batteries with moder-ate voltage. Today, this phase is one of the leading candidates for all-solid-state lithium batteries with durable high energy and high rate performance. However, fundamental knowledge of Li+ The electrochemical reaction of layered titanium disulfide with lithium giving the intercalation compound lithium titanium Disulfide is the basis of a new battery system. The electrochemical reaction of layered titanium disulfide with lithium giving the intercalation compound lithium titanium disulfide is the basis of a new battery system. This reaction occurs very rapidly and in a highly
Electrical Energy Storage and Intercalation Chemistry,Science
In processed form, it is corrosive. We should dispose of lithium-ion batteries responsibly. Whittingham Uses Lithium in a Battery While working for Exxon in the 1970s as a young chemist, Whittingham discovered that lithium ions moved between titanium disulfide cathodes and lithium-aluminum creating electricity. He created the world’s first lithium-ion battery, which sported a titanium disulfide cathode and a lithium anode. The device was lightweight and Following his investigations of the properties of tantalum disulfide, Whittingham and his colleagues made a remarkable discovery. Their breakthrough? Understanding the role of intercalation electrodes in battery reactions. And this would eventually result in the first commercial lithium rechargeable batteries. The batteries were based on a titanium disulfide
Here we investigated the lithiation pathway of titanium disulfide using in situ TEM combined with synchrotron-based pair distribution function measurement and first-principles calculations. Lithium titanium disulfide cathodes Nature Energy 2021-02-19 | Journal article DOI: 10.1038/s41560-020-00765-7
已完结 文献求助详情 标题 Lithium titanium disulfide cathodes 锂钛二硫化物阴极 相关领域 插层(化学) 阴极 材料科学 锂(药物) 二硫键 储能 钛 二硫化钼 化学工程 无机化学 纳米技术 化学 冶金 物理化学 工程类 内分泌学 物理 功率(物理) 医学 量子力学
Vanadium disulfide flakes with nanolayered titanium disulfide coating as cathode materials in lithium-ion batteries The electrochemical reaction of layered titanium disulfide with lithium giving the intercalation compound lithium titanium disulfide is the basis of a new battery system. This reaction occurs very rapidly and in a highly reversible manner at ambient temperatures as a result of structural retention. Titanium disulfide is one of a new generation of solid cathode materials.
清晨好,您是今天最早来到科研通的研友!由于当前在线用户较少,发布求助请尽量完整的填写文献信息,科研通机器人24小时在线,伴您科研之路漫漫前行! 已完结 文献求助详情 Whittingham’s design – a lithium-metal anode, a titanium disulfide cathode and lithium perchlorate in dioxolane as electrolyte – could be charged and discharged thousands of times without suffering performance losses. 3 And most importantly, the battery put out an astonishing 2.5V.
Read this article To access this article, please review the available access options below. Titanium disulfide (Ti 1+x S 2)and trisulfide (TiS 3) were prepared by reaction of the elements. They were characterized by scanning electron microscopy and by X-ray diffraction. Both sulfides are electrochemically active and show good reversibility. Discharge cycles in propylene carbonate and methyl acetate are characterized by relatively flat voltage plateaus at
When the battery discharged, positively-charged lithium ions would migrate from the metallic lithium anode through a non-conductive Whittingham’s battery, the first lithium intercalation battery, was developed at Exxon in 1972 using titanium disulfide for the cathode and metallic lithium for the anode.
His rechargeable battery scheme used a cathode made of titanium disulfide, which contains many layers that can house lithium ions released from the anode. Interestingly, the metal sulfides we found to be most successful (i.e., titanium disulfide and molybdenum disulfide) are also known to be electrochemically active and are hence expected to act as a ‚redox‘ mediator, similar to MnO 2 as reported by Nazar et al. 61 In this paper, we present the performance enhancements achieved with
When lithium is inserted into the van der Waals gap of the layered struc-ture of titanium disulfide there is less than a 10% expansion of the lattice, as shown schematically in Figure 6 (left). On removal of the lithium, the lattice reverts back to its initial state. The perfect reversibility of this reac-tion is known as an intercalation reaction. Here we report that vanadium disulfide flakes can be rendered stable in the electrochemical environment of a lithium-ion battery by conformally coating them with a ~2.5 nm thick titanium disulfide layer.
Here we report that vanadium disulfide flakes can be rendered stable in the electrochemical environment of a lithium-ion battery by conformally coating them with a similar to 2.5 nm thick titanium disulfide layer. 科研通是完全免费的文献互助平台,具备全网最快的应助速度,最高的求助完成率。 对每一个文献求助,科研通都将尽心尽力,给求助人一个满意的交代。 实时播报 秋雪瑶 的 应助 被 DAMAOMI 采纳,获得 10 1秒前 顾矜 上传了 应助文件 3秒前 莫天 驳回了 可爱的函函 的 应助 7秒前 SYHWW
Exxon commercialized the first rechargeable lithium-ion battery, which was based on a titanium disulfide cathode and a lithium-aluminum anode. He developed the hydrothermal synthesis technique for making cathode materials, which is now being used commercially for the manufacture of lithium iron phosphate by Phostech/Sud-Chimie in Montreal, Canada.
- List Of National Football League Finals
- Little Greene. Neuer Auftritt In München
- Live-Webcams Nessebar, Bulgarien ️ Webcamera24
- Live Wallpaper In Ubuntu : Enjoy Animated Wallpaper on Linux
- Liste Der Kanadischen Senatoren Aus Neufundland Und Labrador
- Liste Der Baudenkmale In Schwaan
- Literally Just Rodrick Crying _ Rodrick crying lol #diaryofawimpykid #2000s #funny #viral
- Littlerock Saloon , Littlerock Tavern Menu in Olympia, WA
- Live Music Events In Corpus Christi, Tx
- Live At Bill Graham’S Fillmore West
- Liste Zu Einer Anderen Liste Hinzufügen In C