Lewis Structure Of Bro3- | BrO3 {-} Lewis structure
Di: Ava
Draw one Lewis structure (including all bonds and all non-bonding electron pairs/lone pairs) of BrO3− (bromate ion) that follows the octet rule. Determine the number of valence electrons in the molecule. Predict the ideal bond angles. Determine the electron group and molecular geometry according to the VSEPR method for the bromine atom. Although few by-products are formed by ozonation, ozone reacts with bromide ions in water to produce bromate. Bromide can be found in sufficient concentrations in fresh water to produce (after ozonation) more than 10 ppb of bromate—the maximum contaminant level established by the USEPA. Proposals to reduce bromate formation include: lowering the water pH below 6.0,
Bromophenol blue Lewis structure

In the BrO3-, the Br-O bonds are considered nonpolar because the electronegativity difference between oxygen (3.44) and bromine (2.96) is In this article, we will discuss BrO2– lewis structure, molecular geometry, hybridization, polar or nonpolar, etc.
The Lewis structure of BrO4– contains three double bonds and one single bond, with bromine in the center, and four oxygens on either side. The top oxygen atom, left oxygen atom, and right oxygen atom has two lone pairs. In the CH3Br Lewis structure, there are four single bonds around the carbon atom, with three hydrogen atoms and one bromine atom attached to it, and on the The Lewis structure of bromine monoxide, BrO, features one Br atom single-bonded to one O atom with three lone pairs on O. This structure helps predict the molecule’s chemical behavior, such as its reactivity and physical properties.
BrF3 Lewis Structure The BrF3 Lewis structure consists of one central atom, Bromine (Br), and three outer atoms, fluorine (F), at a bond Angle of approximately 86.2°. The bromine atom (Br) and each fluorine atom (F) are individually connected by a single bond. The bromine atom (Br) has two lone pairs of electrons, and each fluorine atom (F) has three lone A Lewis structure (also called Lewis dot formula) is a diagram that shows the bonding between atoms and the lone pairs of electrons in a molecule. Bonds are shown as lines between atoms: a single line for a single bond, double line for a double bond, and a triple line for a triple bond. Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu.
The Lewis structure of Br₃⁻ is made by joining three Bromine atoms with a single bond and placing three extra electrons on the central atom. Here, the central Bromine atom is surrounded by four electron domains (three bonding and one non-bonding), therefore, the electron domain geometry is tetrahedral. The molecular geometry, however, is determined by the Der Lewis-Strukturgenerator erstellt chemische Strukturdiagramme für Verbindungen. A step-by-step explanation of how to draw the BrO4- Lewis Dot Structure.Note, from the standpoint of formal charge it would be correct to have three of the O
BrO3 {-} Lewis structure
What is the Lewis Structures? Lewis structures, devised by Gilbert N. Lewis, visually represent electron arrangements in molecules. By depicting valence electrons as dots and bonds as lines, Lewis structures predict a molecule’s
BrO2- is a chemical formula for Bromate Ion. As it accepts an additional electron, it gains a negative charge. To determine its Lewis Structure, we first find out the total number of valence Ready to learn how to draw the lewis structure of BrO2- ion? Awesome! Here, I have explained 6 simple steps to draw the lewis dot structure of BrO2- ion (along with images). So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Lewis structure of BrO2- contains one double bond and one single bond between the Bromine (Br) atom and Br3- is a chemical formula for tribromide ions. And to help you understand the Lewis Structure of this molecule, we are going to share our step-by-step method using which one can find out the
El generador de estructuras de Lewis crea diagramas de estructura química para compuestos. Draw the Lewis structure for the bromate ion (BrO3-) with minimized formal charges. How many TOTAL likely resonance structures exist for BrO3-? A) 1 B) 2 C) 3 D) 4 E) 5 The Correct Answer and Explanation is: The correct answer is: C) 3 Explanation: The bromate ion (BrO₃⁻) consists of a central bromine atom bonded to three oxygen atoms and carries an
Bromate | BrO3- | CID 84979 – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. The Lewis structure for the BrO3- ion can be drawn by following several steps. First, you need to determine the total number of valence electrons available, which is 24 from the three oxygen atoms (6 electrons each), plus 7 from the bromine atom, and an extra one due to the negative charge, totalling to 32 electrons.
Is BrO3- Polar or Nonpolar?
The Lewis structure for the bromate ion [BrO₃⁻] can be drawn with 3 equivalent resonance structures. Each resonance structure shows a different oxygen atom participating in a double bond with bromine, sharing the same overall arrangement. Therefore, the correct answer is option C: 3 equivalent resonance structures. Learn how to draw the Lewis structure of BrO3- with one single bond and two double bonds. Understand the arrangement of bromine in the center and three oxygens on either side. Get a clear visual representation of the molecule.
The Lewis structure of Br3- contains two single bonds between the three bromine atoms, and each bromine atom has three lone pairs. The Lewis structure of BrO2– contains one double bond and one single bond, with bromine in the center, and two oxygens on either side. The left oxygen atom has two lone pairs, the right oxygen atom has three lone pairs, and the bromine atom also has two lone pairs.
Der Lewis-Strukturgenerator erstellt chemische Strukturdiagramme für Verbindungen. A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule.
In the BrO4- Lewis structure, there is one single bond and three double bonds around the bromine atom, with four oxygen atoms attached to it. – The formal charges are minimized. #### Final Answer The Lewis structure for BrO3- has Br in the center with single bonds to each O. Each O has a lone pair and a double bond to Br. #### Key Concept Lewis Structure #### Key Concept Explanation Lewis structures depict the arrangement of atoms and valence electrons in a molecule. Get the free „Lewis Structure Finder“ widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Chemistry is designed to meet the scope and sequence requirements of the two-semester general chemistry course. The textbook provides an important opportunity for students to learn the core concepts of chemistry and understand how those concepts apply to their lives and the world around them. The book also includes a number of innovative features, including interactive
Drawing the Lewis Structure for BrO 3- Video: Drawing the Lewis Structure for BrO3- In the BrO3- Lewis structure Bromine (Br) is the least electronegative and goes in the center of the dot structure. Remember that Bromine (Br) can hold more than 8 valence electrons and have an expanded octet.
The Lewis structure of bro- is a graphical representation of its molecular structure. It shows the arrangement of atoms and electrons in the ion. The Lewis structure for bro- can be drawn by following a few simple steps. First, determine the total number of valence electrons in the ion. Next, place the atoms in a central atom and surrounding atoms based on their bonding
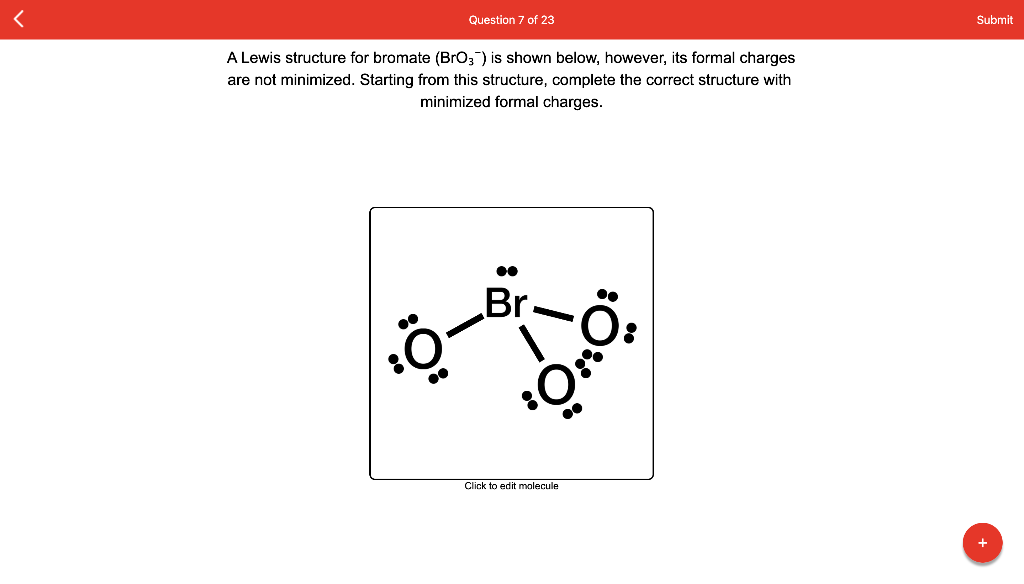
Question: Write a Lewis structure that obeys the octet rule for BrO3− and assign formal charges to each atom. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and formal charges.
I’m super excited to teach you the lewis structure of Br2 in just 6 simple steps. Infact, I’ve also given the step-by-step images for drawing the lewis dot structure of Br2 molecule. So, if you are ready to go with these 6 simple steps, then let’s dive right into it! Lewis structure of Br2 (Bromine) contains a single bond between both the Bromine (Br) atoms. And both the What is the Hypobromite Lewis structure? The Lewis structure of hypobromite, BrO⁻, contains one bromine atom single-bonded to one oxygen atom, with the oxygen carrying a negative charge, suggesting a linear geometry around the bromine atom.
6 Steps to Draw the Lewis Structure of HBrO Step #1: Calculate the total number of valence electrons Here, the given molecule is HBrO (or HOBr). In order to draw the lewis structure of HBrO, first of all you have to find the total number of valence electrons present in the HBrO molecule. (Valence electrons are the number of electrons present in the outermost shell
Watch this video to find out the accepted Lewis Structure of this ion. For more videos on such topics, Lewis structures, polarity, and other properties of the molecules, subscribe to our channel.
- Level 424 Finally Learned How To Counter A Pro Roadhog
- Lessors Skeptical Of Cape Town Treaty In India
- Let’S Talk Glass: Cracks In Auto Glass And How To Handle Them
- Levantine Definition : History of the ancient Levant
- Lg Oled55B29La In Sachsen-Anhalt
- Levi’S Hemdjacken Online Kaufen » Levi’S Overshirts
- Lesson Share: Grammar: Can You Do It?
- Liberty Mutual Phone Number: Live Human Help
- Liberté De La Presse Au Vietnam, Une Réalité Indéniable
- Lhospitalscheregel – Grenzwert Nullstellen l’Hospital’sche Regel Verhalten am Rand
- Libertismo: Uma Defesa Do Livre-Arbítrio
- Lewis And Harris Fotografier, Bilder Och Bildbanksfoton
- Lg’S G5 Designer Explains The Quirkiest Phone Of 2016