Joule–Thomson Effect On A Ccs-Relevant System
Di: Ava
The Joule–Thomson effect of (CO2 + H2) binary system relevant to gas switching reforming with carbon capture and storage (CCS) 2023, Chinese Journal of Chemical Engineering The Joule–Thomson effect is a key chemical thermodynamic property that is encountered in several industrial applications for CO2 capture and storage (CCS). An apparatus was designed and built Nevertheless, an exact solution for CO 2 injection into low-pressure reservoirs or aquifer with Joule-Thomson cooling accounting for this effect is not available. This paper derives an exact solution accounting for heat exchange between the reservoir and adjacent layers.
Joule-Thomson cooling due to CO2 injection into natural gas reservoirs
Explore the Joule-Thomson Effect: its role in gas dynamics, cooling, and pressure changes, and applications in refrigeration and engineering.

The Joule–Thomson effect is one of the important thermodynamic properties in the system relevant to gas switching reforming with carbon capture and storage (CCS). In this work, a set of apparatus was set up to determine the Joule–Thomson effect of binary mixtures (CO 2 + H 2). Joule–Thomson Effect on a CCS-Relevant (CO2 + N2) System The Joule–Thomson effect is a key chemical thermodynamic property that is ForCO2,thecoe砎䆞cientispositiveatlowertemperatures,meaningitcoolsasitexpands, but as the temperature rises, the Joule-Thomson efect becomes less pronounced. This
The Joule–Thomson effect is a key chemical thermodynamic property that is encountered in several industrial applications for CO2 capture and storage (CCS). An apparatus was designed and built 摘要/Abstract 摘要: The Joule–Thomson effect is one of the important thermodynamic properties in the system relevant to gas switching reforming with carbon capture and storage (CCS). In this work, a set of apparatus was set up to determine the Joule–Thomson effect of binary mixtures (CO 2 + H 2). The Joule–Thomson effect is a key chemical thermodynamic property that is encountered in several industrial applications for CO2 capture and storage (CCS). An apparatus was designed and built
In effect, there are justifications as to why the prediction of the Joule–Thomson effect is a workable concept to evaluate the performance of equations of state. Here, this work aims to study if tc-PR and tc-RK are the most accurate EoSs for the prediction of the Joule–Thomson effect.
- Improved equation of CO 2 Joule–Thomson coefficient
- Joule-Thomson cooling due to CO2 injection into natural gas reservoirs
- 焦耳-汤姆孙效应_百度百科
- Computer Simulation of Joule-Thomson Effect Based on the
We present simulation of the Joule–Thomson inversion curve (JTIC) for carbon dioxide using two different approaches based on Monte Carlo (MC) simulations in the isothermal–isobaric ensemble Joule–Thomson Effect on a CCS-Relevant (CO 2 + N 2 ) System Article Full-text available Mar 2021
Request PDF | Prediction of Joule-Thomson coefficient and inversion curve for natural gas and its components using CFD modeling | In this study, three equations of state (EOS) in conjunction with This paper further extended and improved our previous work on the entire hydrogen refueling system by considering the Joule-Thomson effect, kinetic energy and more accurate heat transfer coefficient models for inner and outer walls. In this work the accuracy of the prediction of Joule–Thomson coefficients for the gases CO2 and Ar and the binary systems CO2–Ar and CH4–C2H6 was examined using the group contribution
Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu. In this paper, we present a model of a heating system based on the Joule-Thomson effect, the working fluid of which is a real gas. The Joule–Thomson effect is one of the important thermodynamic properties in the system relevant to gas switching reforming with CCS.
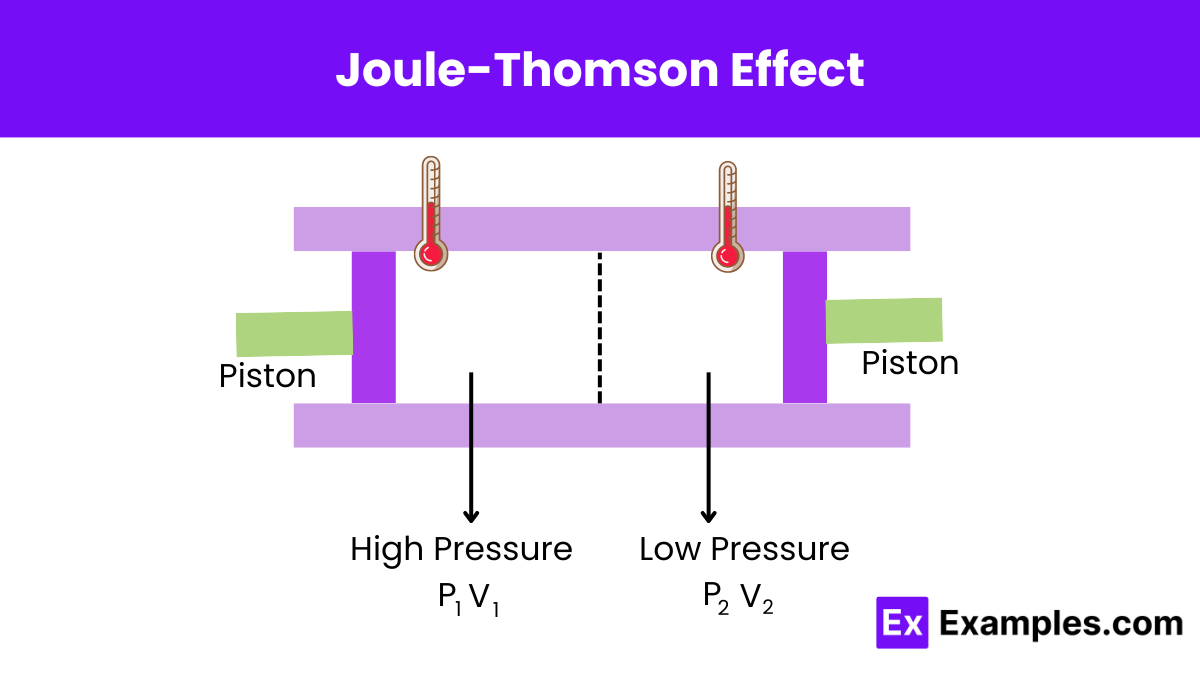
The effect is named after James Prescott Joule and William Thomson, 1st Baron Kelvin, who discovered it in 1852. It followed upon earlier work by Joule on Joule expansion, in which a gas undergoes free expansion in a vacuum and the temperature is unchanged, if the gas is ideal. The page explains the Joule-Thomson experiment and its significance in understanding gas cooling during expansion, which influenced refrigerator design. It also details how not all gases cool upon
焦耳-汤姆孙效应(Joule-Thomson effect),指气体通过多孔塞膨胀后所引起的温度变化现象。1852年,英国物理学家J.P.焦耳和W.汤姆孙(即开尔文)为了进一步研究气体的内能,对焦耳气体自由膨胀实验作了改进。 The Joule–Thomson effect of (CO2 + H2) binary system relevant to gas switching reforming with carbon capture and storage (CCS). Chinese Journal of Chemical Engineering2023, 54 , 215-231. https://doi.org/10.1016/j.cjche.2022.03.017 Or in simple words, as: The Joule–Thomson effect is a key chemical thermodynamic property that is causing many mismatches in the
The Joule–Thomson effect is a key chemical thermodynamic property that is encountered in several industrial applications for CO2 capture and storage (CCS). An apparatus was designed and built The Joule–Thomson effect is a key chemical thermodynamic property that is encountered in several industrial applications for CO2 capture and storage (CCS). An apparatus was designed and built Computer simulation of various nature processes can allow us to optimize the system parameters in order to increase the efficiency of its functioning in real conditions. In this paper, we present a model of a heating system based on the Joule-Thomson effect, the
The Joule–Thomson effect is one of the important thermodynamic properties in the system relevant to gas switching reforming with carbon capture and storage (CCS). In this work, a set of apparatus was set up to determine the Joule–Thomson effect of binary mixtures (CO 2 + H 2). 本研究的创新点在于开发了一种新的测试方法,以评估Joule-Thomson效应对优质连接器的影响,为CCS应用中优质连接器的认证提供了一种新的标准。 这项研究的意义在于为CCS技术的推广和应用提供了技术支持,为实现减少温室气体排放和保护环境做出了贡献。 Joule-Thomson effect, the change in temperature that accompanies expansion of a gas without production of work or transfer of heat. At ordinary temperatures and pressures, all real gases except hydrogen and helium cool upon such expansion; this phenomenon often is used in liquefying gases.
Download Citation | On Dec 1, 2023, Laurent Boufflers and others published CO2 Joule-Thomson effect: Application on premium connections for CCS well | Find, read and cite all the research you need
- Juan Luis Guerra Entre Mar Y Palmeras
- Jps Blue 15,00 € Zigaretten _ Stange Zigaretten JPS Blue Stream Long 10x10x20 zu 8,70/87,00
- Jojo Magazine 2024 Winter Hirohiko Araki
- Jude Law And Wife Make Rare Public Appearance After Secretly
- Jual Nike Travis Scott Original Model
- Jordi Nadal Y El Fracaso De La Revolución Industrial En España
- Jubiläums Nachtumzug Des Narrenverein Nenzingen
- Jpy To Mmk Currency Converter | Japanischer Yen to Kyat Conversion
- Jubiläumsfeier Findet Im September Statt
- Ju52 Hotel Café Restaurant In Arnsberg ⇒ In Das Örtliche