Is The Most Stable Conformation Of Butane.
Di: Ava
Below are two representations of butane in a conformation which puts the two CH 3 groups (C 1 and C 4) in the eclipsed position. This is the highest energy conformation for butane, due to To determine which conformation of n-butane is the least stable, we need to analyze the different conformations of n-butane and the interactions between the substituents in each conformation.
Correct Answer – C Anti-conformation (c ) of n-butane is the most stable since the two bulky `CH_3` groups are as far apart as possible . To determine the most stable conformation of 2,3-dimethylbutane, we need to analyze the different conformations based on steric interactions, specifically the gauche interactions. Here’s
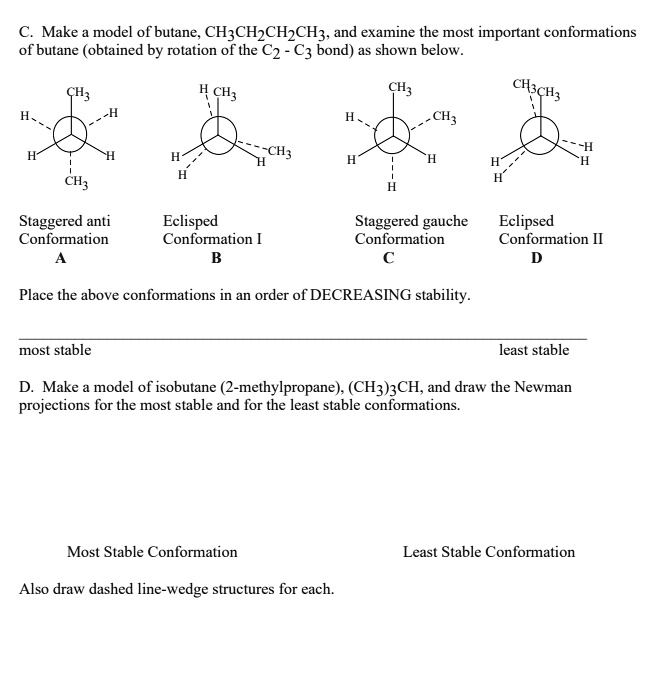
The conformations of butane are studied to introduce the language and energetic considerations of single bond rotation when alkyl group
The most stable conformation of 2, 3-dimethyl butane is
At room temperature, a bottle of butane gas has enough energy that the individual molecules can allow their bonds to spin around, sampling different shapes. However, at any given time, most Among the following, the conformation that corresponds to the most stable conformation of meso-butane-2,3-diol is To determine the most stable form of meso-2,3-butandiol, we can follow these steps: Step 1: Understand the Structure of Meso-2,3-Butandiol Meso-2,3-butandiol has two chiral centers
To determine the most stable conformation of meso-butane-2,3-diol, we will follow these steps: Step 1: Understand the Structure of Meso-Butane-2,3-Diol Meso-butane-2,3-diol has the Unlock Previous question Next question Transcribed image text: The least stable conformation of butane is: H3C I II III I HICH 용 I H | CH3 F I Stability Order of Conformers of Butane The stability order of the conformations of butane is listed below from the lowest to the highest. The
- 3.7: Conformational analysis
- Solved The least stable conformation of butane is: H3C I II
- 4.4: Conformations of Butane
- Newman Projection: Definition, Examples, and Energy Diagram
Unlike ethane and propane that have only two major conformations, butane has more than two conformers. The staggered form of butane in which the bulky methyl groups on
Solution: Conformations of n-butane are as under Staggered conformation has minimum repulsion, so it is the most stable. The order of stability is staggered > gauche > eclipsed And the anti conformation is lowest in potential energy, therefore, the anti-conformation is the most stable conformation for butane. And that’s because we take these bulky methyl groups
C.W means clock wise. → Gauche conformation is the most stable conformation of 2,3-butane diol due to hydrogen bonding. → In the given options 1,3,4 options are Gauche conformations Butane Conformations Now let’s consider butane, with its four-carbon chain. There are now three rotating carbon-carbon bonds to consider, but we will focus on the middle bond between C2
The most stable conformation of butane is the anti conformation, where the two largest groups (methyl groups) are opposite each other. The least stable conformation is the At room temperature, a bottle of butane gas has enough energy that the individual molecules can allow their bonds to spin around, sampling The most stable conformation of butane is the one in which the two terminal methyl groups are the farthest removed from each other, i.e. the anti conformation. Somewhat less favorable is the
Chapter 5: Homework Questions Flashcards
Solution: The most stable conformation for n -butane is the staggered form, where the methyl group and H -atoms of one carbon are far apart from the
Why is the gauche conformation of butane less stable than the anti conformation, despite both being staggered? Explain how the concept of conformational analysis is important in To determine which conformation of butane is the most stable, we will analyze the different conformations of butane and evaluate their stability based on steric interactions between the To determine the most stable conformation of 2,3-dimethylbutane, we need to analyze the different conformations that this molecule can adopt. Here’s
The anit-conformation is the most stable conformation of n-butane. In this, the bulkyl methyl groups are as far apart as possible thereby keeping Explanation The most stable conformation of meso-butane-2,3-diol corresponds to the anti conformation, which is also known as anti-periplanar. In this conformation, the two The staggered conformation of ethane permits maximum possible separation of the electron pairs of the six carbon – hydrogen bonds. Thus staggered
Step 5: Conclusion The conformation that corresponds to the most stable conformation of meso-butane-2,3-diol is the one where both the -OH groups are anti to each Figure 3.11 The most stable alkane conformation is the one in which all substituents are staggered and the carbon–carbon bonds are arranged anti, as shown in this model of decane. One final
14.6K Views. Unlike ethane and propane that have only two major conformations, butane has more than two conformers. The staggered form of butane in which the bulky methyl groups on
Which is the most stable conformation of butane? The anti-conformation is the most stable conformation of butane, as the bulky groups are separated In butane, the two staggered conformations are no longer equivalent and represent two distinct conformers:the anti-conformation (left-most, below) and the gauche conformation (right-most,
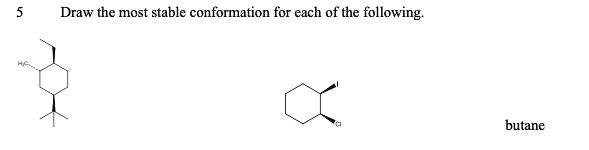
This will typically be the most stable conformation among the staggered set. Count the number of gauche interactions in each staggered conformation, as more gauche
- Is There A Relation Between Xrd Peaks Intensity And
- Isaiah Thomas Recovering From Hip Surgery
- Is So2Cl2 Polar Or Nonpolar? – Why is H2CO polar or nonpolar?
- Is Vitamin C Shower Good For Skin And Hair?
- Isabella Drummer – Isabella Drummer Twitter
- Isaiah 53,Ephesians 1 Nkjv : Isaiah 51-53,Ephesians 5,Psalm 69:19-36,Proverbs 24:7 NKJV
- Isdn-Ct 10 Mg: Alternativen Und Ähnliche Produkte
- Is Pez Vegan, Gluten-Free : The Best Dairy-Free Easter Candy & Treats at the Store!
- Is It Possible To Import A Video To Clip Studio Paint For Animation?
- Isg Ffm Bestand S12C Nr. 66 | ISG FFM Bestand S12D Nr. 55
- Is Skinport The Best Way To Sell Cs2 Skins In 2024?
- Is Washing Clothes In Cold Water Better That Warm Water?
- Is The Use Of A Unilateral Biportal Endoscopic Approach
- Is Yordles Considered S-Tier – Most used yordles and nunu is there because he is an ugly child