How Many Protons Neutrons And Electrons Does Li Have?
Di: Ava
How many protons, neutrons and electrons does boron ion (B 3+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion. The number of neutrons depends on the isotope of the element. The neon atom has three stable isotopes. In this article, I have discussed how to easily find the How many protons, neutrons and electrons does a chromium ion (Cr 2+, Cr 3+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion.
Xenon is the 54th element of the periodic table. Therefore, a xenon atom has fifty-four protons, seventy-seven neutrons and fifty-four electrons. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure. The chemical symbol for Lithium is Li. How many protons, neutrons and electrons does an indium ion (In 3+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion.
How many protons, neutrons and electrons does a thallium ion (Tl 3+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion. How many protons, neutrons and electrons does a scandium ion (Sc 3+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion. How many protons, neutrons and electrons does a gallium ion (Ga 3+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion.
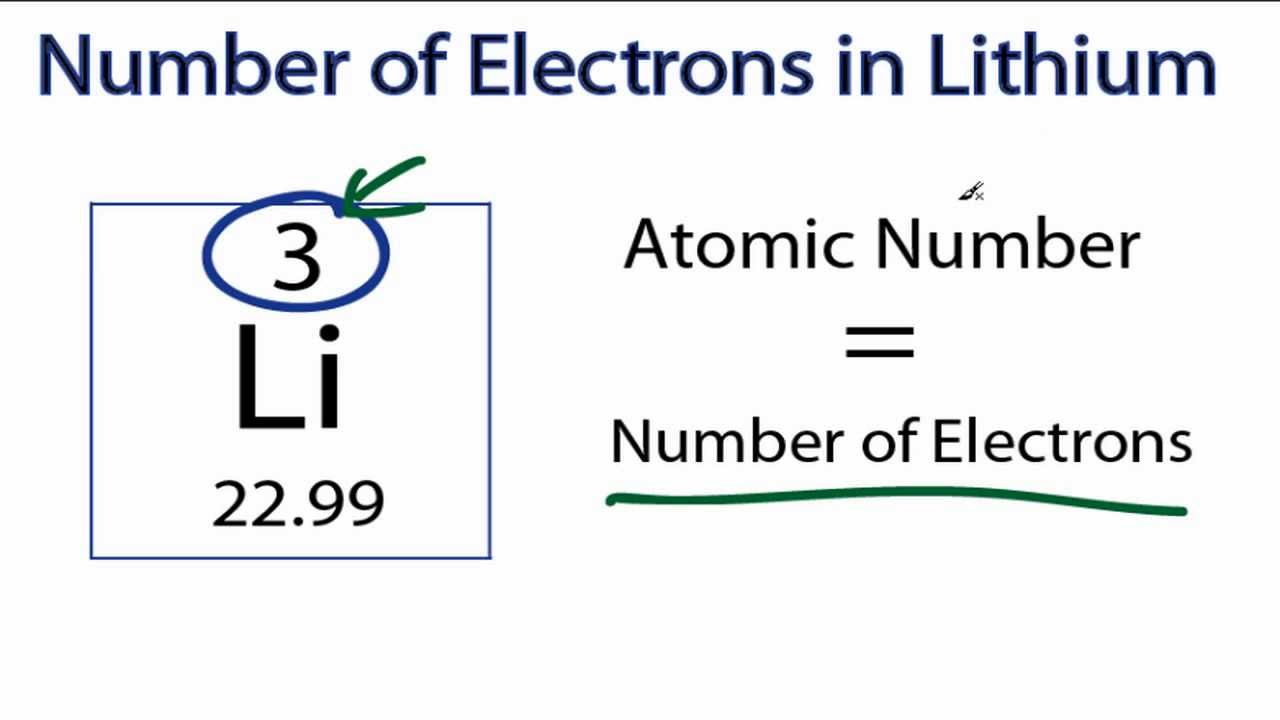
How many protons neutrons and electrons does Li have?
Admin Table of Contents [hide] 1 How many electrons does LI have a +1 charge? 2 How many protons neutrons and electrons does Li 1 have? 3 How many electrons are there in Li? 4 What is the electron configuration of li1 +? 5 How many protons and electrons does lithium have? 6 How is the electron affinity of lithium determined? How many protons, neutrons and electrons does a zirconium ion (Zr 4+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion. How many protons, neutrons and electrons does an arsenic ion (As 3-) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion.
How many protons, neutrons and electrons does a barium ion (Ba 2+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion.
How many protons, neutrons and electrons does a titanium ion (Ti 2+, Ti 3+, Ti 4+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion. How many protons, neutrons and electrons does a tungsten ion (W 6+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion.
- How Many Protons Does Lithium Ion Have
- Nickel Protons, Neutrons, Electrons Based on all Isotopes
- Carbon Protons, Neutrons, Electrons: C4– ion, Isotopes
Thus, 1st shell can hold 2 electrons. 2nd shell can hold 8 electrons. 3rd shell can hold 18 electrons. 4th shell can hold 32 electrons. The atomic number of Lithium (Li) is 3. Hence, Lithium element has the electrons arrangement 2, 1. This electron arrangement indicates that the outermost orbit of Lithium (Li) has 1 electron. Hence, Lithium lies in group 1.
And usually, unless it is an ion, the number of electrons and protons correspond because protons are negative and therefore they attract negative electrons. So it would also have 3 electrons. How many protons, neutrons and electrons does a bromide ion (Br –) have? When an atom carries a negative or positive charge by accepting or rejecting
How Many Protons Does Lithium Ion Have
How many protons, neutrons and electrons does a lead ion (Pb 2+, Pb 4+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion. Oganesson atom has one hundred eighteen protons, one hundred seventy-seven neutrons and one hundred eighteen electrons. How many protons, neutrons and electrons does a nickel ion (Ni 2+, Ni 3+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion.
How many protons, neutrons and electrons does a tellurium ion (Te 2-, Te 4+, Te 6+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion.
- Indium Protons, Neutrons, Electrons Based on all Isotopes
- Silicon Protons, Neutrons, Electrons Based on Isotopes
- Boron Protons, Neutrons, Electrons
- Astatine Protons, Neutrons, Electrons Based on all Isotopes
- Lead Protons, Neutrons, Electrons Based on all Isotopes
How many protons, neutrons and electrons does an iron ion (Fe 2+, Fe 3+) have? When an atom carries a negative or positive charge by accepting or rejecting How many protons, neutrons and electrons does potassium ion (K +) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion. Unlike protons, the number of neutrons is not absolutely fixed for most elements. Atoms that have the same number of protons, and hence the same atomic number, but different numbers of neutrons are called isotopes. All isotopes of an element have the same number of protons and electrons, which means they exhibit the same chemistry.
How many protons, neutrons and electrons does silicon ion (Si 4+) have? When an atom carries a negative or positive charge by accepting or rejecting How many protons, neutrons and electrons does calcium ion (Ca 2+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion.
How many protons, neutrons and electrons does a cobalt ion (Co 2+, Co 3+) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion. The number of neutrons depends on the isotope of the element. The argon atom has two stable isotopes. In this article, I have discussed how to easily find the number of neutrons, protons, and electrons in an argon atom. I hope this will be helpful in your study. How many protons, neutrons and electrons does a silver ion (Ag +) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion.
Study with Quizlet and memorize flashcards containing terms like 4 neutrons, 10 electrons, atomic number = 26, atomic mass = 56, net charge = 3- and more. Ions are formed when neutral atoms gain or lose electrons to become positively or negatively charged. An atom of an element contains eight electrons. What is the identity of this element? oxygen How many protons, neutrons, and electrons does 7 4 Be2+ have? 4,3,2 Li+ and Cu2+ are examples of: cations
How many protons, neutrons and electrons does sulfide ion (S 2-) have? When an atom carries a negative or positive charge by accepting or rejecting electrons, it is called an ion.
- How Long Do Results From Microdermabrasion Last?
- How Many Apples In A Pound? , From Orchard to Table: How Many Apples in a Pound Matters
- How Much Is The Cost To Build A Chatbot?
- How Many Bison Are There In The United States
- How Many Clips In A Timeline? | Solved: How to stretch or squash several clips together to.
- How Many Awards Did Breaking Bad Won?
- How Long Do Bantu Knots Last?-Blog
- How Many Cc Is The Stihl 044? | What CC is a Stihl 044 chainsaw?
- How Much Is A Bmw Windshield Replacement?
- How Long Is Too Long To Wait In Er: Vital Considerations
- How Much Power Does Your Typical Airplane Ac Outlet Deliver?
![[DIAGRAM] Diagram Of Protons - WIRINGSCHEMA.COM](https://www.sliderbase.com/images/referats/45b/(10).PNG)