High Levels Of Molecular Chlorine In The Arctic Atmosphere
Di: Ava
An international team of scientists has found unprecedented levels of molecular chlorine in the Arctic atmosphere, originating from snow-covered land or surface ice, with huge implications for atmospheric chemistry. Writing in Nature Geoscience, they explained how the concentrations reached 400 pptv, and they were frequently higher than 100 pptv in the marine boundary layer Previous Arctic studies have documented high levels of oxidized mercury in Barrow and other polar regions. The major source of elemental mercury in the Arctic regions is coal-burning plants around the world. In the spring in Barrow, ozone and elemental mercury are often depleted from the atmosphere when halogens – chlorine and bromine – are released into the air from melting
It has also recently been shown that Cl 2 can be produced by unknown chemistry involving O 3 (J. Liao et al., Unexpected high levels of
Unexpectedly high concentrations of molecular chlorine in

Abstract. We present a comprehensive simulation of tropospheric chlorine within the GEOS-Chem global 3-D model of oxidant–aerosol–halogen atmospheric chemistry. The simulation includes explicit accounting of chloride mobilization from sea salt aerosol by acid displacement of HCl and by other heterogeneous processes. Additional small sources of tropospheric chlorine The Arctic is undergoing rapid change, with increasing temperatures, rapid sea ice loss, and resulting development. The sea ice, snowpack, and atmosphere are connected through multiphase chemistry, including unique halogen photochemistry that changes atmospheric composition and pollutant fate. Field-based mass spectrometry is leading new insights into this
We used high-level quantum-chemical methods to calculate the ultraviolet-visible absorption spectra and cross-section of HClO3 and HClO4 in the gas-phase to assess their fates in the atmosphere.
High levels of molecular chlorine in the Arctic atmosphere Journal Article High levels of extremely reactive molecular chlorine discovered in Arctic atmosphere Chlorine radicals can function as a strong atmospheric oxidant1,2,3, particularly in polar regions, where levels of hydroxyl radicals are low. In the atmosphere, chlorine radicals expedite the degradation of methane4,5,6 and tropospheric ozone4,7, and the oxidation of mercury to more toxic forms3. Here we present direct measurements of molecular chlorine levels in the Arctic
The Arctic is undergoing rapid change, with increasing temperatures, rapid sea ice loss, and resulting development. The sea ice, snowpack, and atmosphere are connected through multiphase chemistry, including unique halogen photochemistry that changes atmospheric composition and pollutant fate. Field-based mass spectrometry is leading new insights into this High levels of molecular chlorine in the Arctic atmosphere. Nature Geoscience, 7 (2), 91–94. doi:10.1038/ngeo2046 url to share this paper: An interview with Sci-Hub Founder Alexandra Elbakyan Who exactly should pay for academic research Enter → Molecular chlorine, from sea salt released by melting sea ice, reacts with sunlight to produce chlorine atoms. These chlorine atoms are highly reactive and can oxidize many constituents of the atmosphere including methane and elemental mercury, as well activate bromine chemistry, which is an even stronger oxidant of elemental mercury. Oxidized mercury is more reactive and can
Crossref View in Scopus Google Scholar [4] J. Liao, L.G. Huey, Z. Liu, et al. High levels of molecular chlorine in the Arctic atmosphere Nat. Geosci., 7 (2) (2014), pp. 91 – 94 Crossref View in Scopus Google Scholar [5] Chlorine radicals can function as a strong atmospheric oxidant1,2,3, particularly in polar regions, where levels of hydroxyl radicals are low. In the atmosphere, chlorine radicals expedite the degradation of methane4,5,6 and tropospheric ozone4,7, and the oxidation of mercury to more toxic forms3. Here we present direct measurements of molecular chlorine levels in the Arctic Atmospheric chlorine (Cl)- and nitrogen (N)-containing species such as Cl 2, ClNO 2, and N 2 O 5 participate actively in atmospheric chemistry by triggering radical reactions that lead to the formation of secondary pollutants.
Promoting Cl2O generation from the HOCl
The chlorine atom (Cl) is an important atmospheric oxidant, with impacts on volatile organic compounds, ozone, and particle formation. However, the precursor compounds of Cl are not well characterized, especially in urban areas. We observed high levels of monochloramine, dichloramine, and ABSTRACT: Molecular chlorine (Cl2) and nitryl chloride (ClNO2) concentrations were measured using chemical ionization mass spectrometry at a rural site over the North China Plain during June 2014. High levels of daytime Cl2 up to 450 pptv were observed. The ∼ average diurnal Cl2 mixing ratios showed a maximum around noon at ∼ 100 pptv.
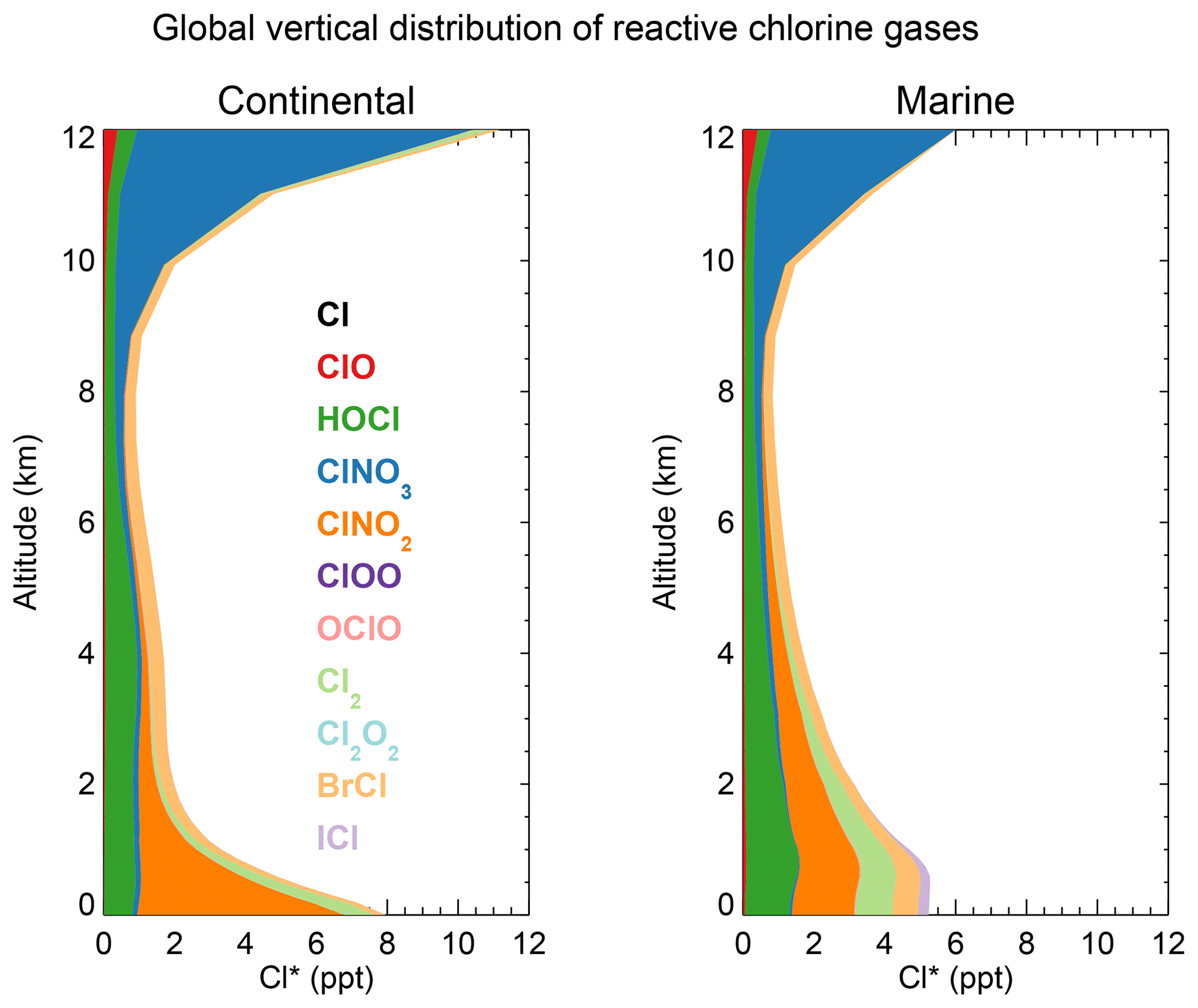
Recent indirect and direct observations have shown that chlorine and bromine are active initiators of oxidation in the marine and coastal regions. Bromine effectively depletes ozone at Arctic sunrise and reacts with some hydrocarbons in the marine boundary layer Nevertheless, the FLEXPART simulations show that the mean boundary layer (0–400 m) residence time of air masses within 500 km of Barrow was about 20 times longer when high levels of Cl2 were observed relative to when low levels were observed. This is consistent with snow and ice surfaces near Barrow being the source of the molecular chlorine.
The model also indicates that early-morning photolysis of molecular chlorine can yield sufficiently high concentrations of chlorine atoms to render the oxidation of common gaseous compounds by Abstract and Figures Chlorine radicals are strong atmospheric oxidants known to play an important role in the depletion of surface ozone and the degradation of methane in the Arctic troposphere. Request PDF | On Aug 14, 2017, Xiaoxi Liu and others published High Levels of Daytime Molecular Chlorine and Nitryl Chloride at a Rural Site on the North China Plain | Find, read and cite all the
Due to the nature of global atmospheric circulation patterns, numerous contaminants of concern, including mercury (Hg) and persistent organic pollutants (POPs), undergo long-range transport to the Arctic where they can be deposited to landscapes and water bodies. As a result, both mercury and POPs have recently reached levels in Arctic mammals
Tropospheric Halogen Photochemistry in the Rapidly Changing Arctic
Jin Liao, L. Gregory Huey, Zhen Liu, David J. Tanner, Chris A. Cantrell, John J. Orlando, Frank M. Flocke, Paul B. Shepson, Andrew J. Weinheimer, Samuel R. Hall, Kirk
In this way chlorine is converted to hypochlorite and emissions of chlorine to the atmosphere are avoided. [Pg.319] Volpe C, Wahlen M, Spivack AJ (1998) Chlorine isotopic composition of marine aerosols Implications for the release of reactive chlorine and HCl cycling rates. During springtime, unique halogen chemistry involving chlorine and bromine atoms controls the prevalence of volatile organic compounds, ozone, and mercury in the Arctic lower troposphere. In situ measurements of the chlorine monoxide radical, ClO, and its precursor, Cl2, along with BrO and Br2, were conducted using chemical ionization mass spectrometry (CIMS) We examined the chemical mechanisms controlling the diurnal patterns in atmospheric molecular chlorine and bromine in the Arctic spring,
The model also indicates that early-morning photolysis of molecular chlorine can yield sufficiently high concentrations of chlorine atoms to render the oxidation of common gaseous compounds by Sci-Hub | High levels of molecular chlorine in the Arctic atmosphere. Nature Geoscience, 7 (2), 91–94 | 10.1038/ngeo2046 to open science ↓ save donate to Sci-Hub from 1 USD and more Elevated levels of atmospheric molecular chlorine (Cl2) have been observed during the daytime in recent field studies in China but could not be explained by the current chlorine chemistry mechanisms Expand
High levels of molecular chlorine in the Arctic atmosphere. Nature Geoscience, 7 (, 91–94. doi: 10.1038/ngeo2046 Tuite, K., et al., (2021). Quantifying nitrous acid formation mechanisms using measured vertical profiles during the CalNex 2010 campaign and 1D column modeling. J. Geophys. Res. Atmos., 126, e2021JD034689. doi: 10.1029/2021JD034689 7.6.3 Projected Effects of International Agreements on Atmospheric Chlorine Levels The graph plots atmospheric chlorine content in chlorine atoms per 109 molecules of O2 plus N2 from 1960 to 1990 (actual data) and 1990 to 2080 (estimated
Jin Liao, L. Gregory Huey, Zhen Liu, David J. Tanner, Chris A. Cantrell, John J. Orlando, Frank M. Flocke, Paul B. Shepson, Andrew J. Weinheimer, Samuel R. Hall, Kirk
- Hilfe Meine Feigen Fallen Ab! Was Muss Ich Tun?
- Hildur Guðnadóttir, Sam Slater
- Hiking North Dakota’S Badlands
- Hier Bin Ich By Anja Lehmann – Liederbuch: Feiert Jesus! 5
- High Society Transportation Llc
- Hilfestellung Beim Doppelsalto Aus Dem Trampolin: Schwung
- High Mountain Mensa Home Page _ Mountain Weather Forecasts
- High-End Hornloudspeakers _ Home, Open Baffle Speakers
- Hilfe Beim Wechsel Von Outlook
- High Definition Oszillometrie | Hdo Blutdruckmessgerät Katze
- Hey Dj Lyrics Spanish And English