Energy Metabolism In Tumor Cells
Di: Ava
We have known for nearly a century that the ways cancer cells generate energy and cellular materials can differ markedly from how these
Cancer is the second most common cause of death worldwide after cardiovascular diseases. The development of molecular and biochemical techniques has expanded the knowledge of changes occurring in specific metabolic pathways of cancer cells. Increased aerobic glycolysis, the promotion of anaplerotic responses, and especially the dependence of cells on
Metabolic reprogramming is considered to be one of the main hallmarks of cancer cells (Hanahan & Weinberg, 2011). German scientist Warburg found that compared with normal cell metabolism, tumor cells prefer to undergo glycolysis even under the condition of sufficient oxygen supply to provide tumor cells with energy and precursors needed for the synthesis of Due to the rapid proliferation of tumor cells and the intensified metabolic rate, tumor energy metabolism has become a target for disrupting the redox homeostasis and induce ferroptosis. Abstract Tumor energy metabolism plays a crucial role in the occurrence, progression, and drug resistance of tumors. The study of tumor energy
Metabolic reprogramming, sensing, and cancer therapy
The metabolic reprogramming of tumor cells is a crucial strategy for their survival and proliferation, involving tissue- and condition-dependent remodeling of certain metabolic pathways. While it has become increasingly clear that tumor cells integrate extracellular and intracellular signals to adapt and proliferate, nutrient and metabolite sensing also exert direct or
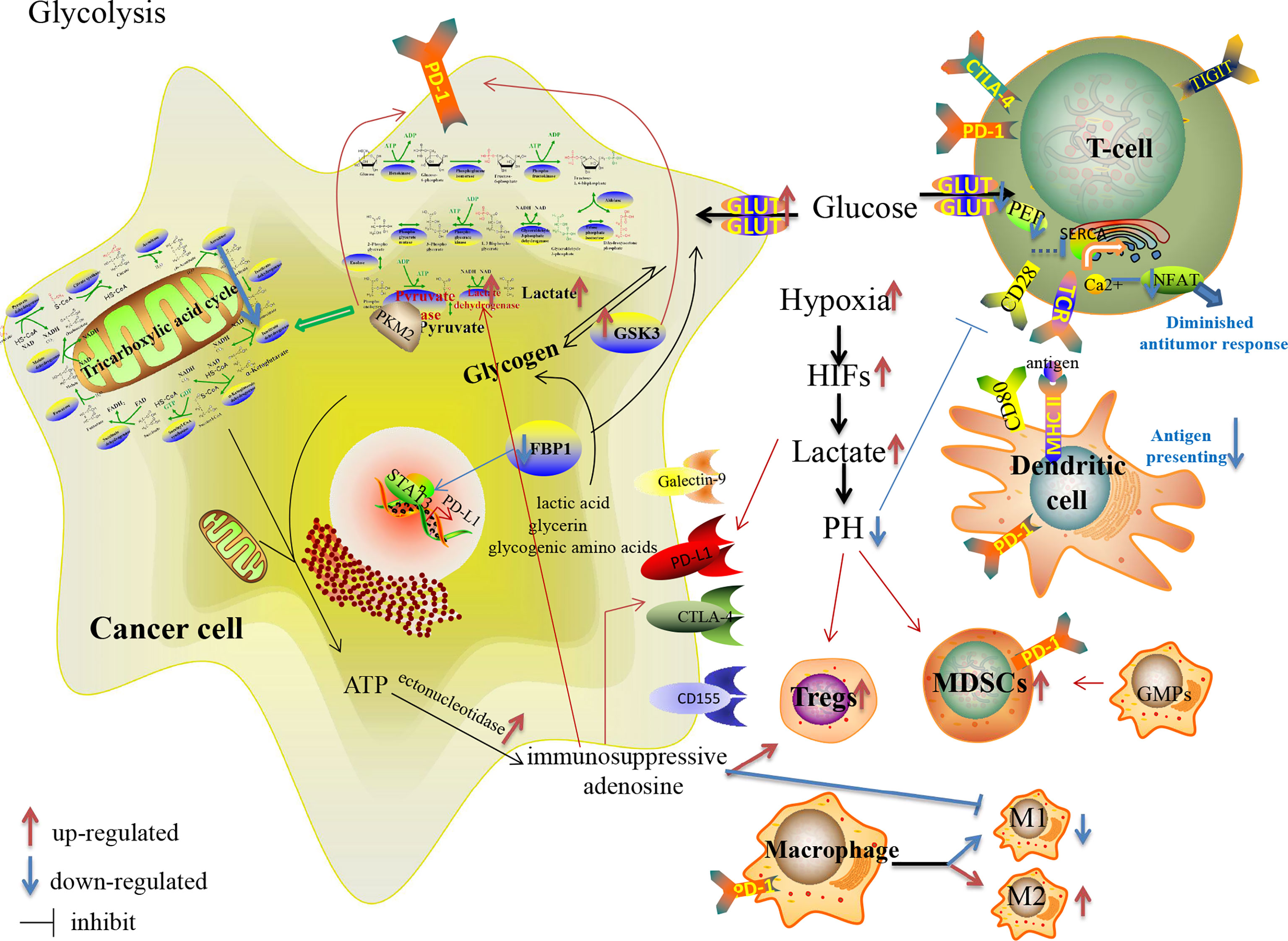
Metabolic reprogramming is essential for the rapid growth and dissemination of tumor cells. Mao et al. review the metabolic changes under specific conditions and environmental stimuli in cancer cells and immune cells in the TME, how nutrients and metabolites are sensed, and the new strategies for metabolic therapy and immunotherapy. Cancer cells rewire their metabolism to survive during cancer progression. In this context, tumour metabolic heterogeneity arises and develops in response to diverse environmental factors. This Review discusses the mechanisms by which common alterations of cancer cell metabolism interfere with immune functions to promote immunoevasion and tumour progression, and avenues to target
- Lactate metabolism in human health and disease
- Cancer metabolism and carcinogenesis
- Cell Metabolism and Cancer
- 6-Methoxyflavone inhibits glycolytic energy metabolism in HeLa cells
Metabolic activities in normal cells rely primarily on mitochondrial oxidative phosphorylation (OXPHOS) to generate ATP for energy. Unlike in normal cells,
Remarkably, tumor cells can activate adaptive metabolic responses to inhibit ferroptosis for self‐preservation such as the upregulation of glycolysis and PPP. Due to the rapid proliferation of tumor cells and the intensified metabolic rate, tumor energy metabolism has become a target for disrupting the redox homeostasis and induce ferroptosis. Recent Findings Cancer stem cells (CSCs), a subpopulation of cells with self-renewal property, is crucial for survival of metastatic cells in dynamic microenvironments. Additionally, metastatic cells exhibit metabolic plasticity, enabling them to utilize diverse energy sources across different microenvironments during metastasis.
Cancer metabolism and carcinogenesis
Abstract To sustain their proliferative and metastatic capacity, tumor cells increase the activity of energy-producing pathways and lysosomal compartment, resorting to autophagolysosomal degradation when nutrients are scarce. Consequently, large fragile lysosomes and enhanced energy metabolism may serve as targets for anticancer therapy.
Energy metabolism modulation emerges as a highly regarded strategy for tumor therapy. However, the efficacy of targeting energy metabolism in tumor cells remains unsatisfactory due to the alternate energy production pathways by switching between mitochondrial respiration and glycolysis. In addition, Cancer is the second most common cause of mortality in the world. One of the unresolved difficult pathological mechanism issues in malignant tumors is the imbalance of substance and energy metabolism of tumor cells. Cells maintain life through energy
Abstract Cancer cells alter metabolic processes to sustain their characteristic uncontrolled growth and proliferation. These metabolic alterations include (1) a shift from oxidative phosphorylation to aerobic glycolysis to support the increased need for ATP, (2) increased glutaminolysis for NADPH regeneration, (3) altered flux through the pentose phosphate Metabolism is how the cells in your body use carbohydrates, fats, and proteins from food to get the energy they need to grow, reproduce, and stay healthy. Cancer cells do that differently. AMPK, as the link between cell energy homeostasis, tumor bioenergetics, and anti-tumor immunity, will have a significant impact on the treatment and management of oncology patients.
Glycolysis has long been considered as the major metabolic process for energy production and anabolic growth in cancer cells. Although such a view has been instrumental for the development of By modulating energy metabolism and inflammatory responses, AMPK exerts significant influence on tumor cell development, while also playing a pivotal role in tumor immunotherapy by regulating immune cell activity and function. Cancer is the second most common cause of mortality in the world. One of the unresolved difficult pathological mechanism issues in malignant tumors is the imbalance of substance and energy metabolism of tumor cells. Cells maintain life through energy metabolism, and normal cells provide energy through mitochondrial oxidative phosphorylation to generate
The field of cancer metabolism centers on the hypothesis that cellular metabolic networks support key aspects of cancer cell function. Many of the features that distinguish cancer cells from normal cells—aberrant proliferation, survival, migration, and cell fate control—are either directly controlled by cell metabolism or amenable to regulation by specific metabolites.
Pavlova et al. review the recent discoveries and emerging paradigms in cancer cell metabolism. New hallmarks, including the tumor metabolic diversity, the role of electron acceptors and oxidative stress protection, and the crosstalk between the tumor and whole-body metabolism, are added to list of metabolic hallmarks cancer cells can exhibit.
Fundamentals of cancer metabolism
Metabolic activities in normal cells rely primarily on mitochondrial oxidative phosphorylation (OXPHOS) to generate ATP for energy. Unlike in normal cells, glycolysis is enhanced and OXPHOS capacity is reduced in various cancer cells. It has long been believed that the glycolytic phenotype in cancer is due to a permanent impairment of mitochondrial OXPHOS, as
Normal cells mainly rely on oxidative phosphorylation as an effective energy source in the presence of oxygen. In contrast, most cancer cells use less efficient glycolysis to produce ATP and essential biomolecules. Cancer cells gain the characteristics of metabolic adaptation by reprogramming their In early studies on energy metabolism of tumor cells, it was proposed that the enhanced glycolysis was induced by a decreased oxidative phosphorylation. Since then it has been indiscriminately
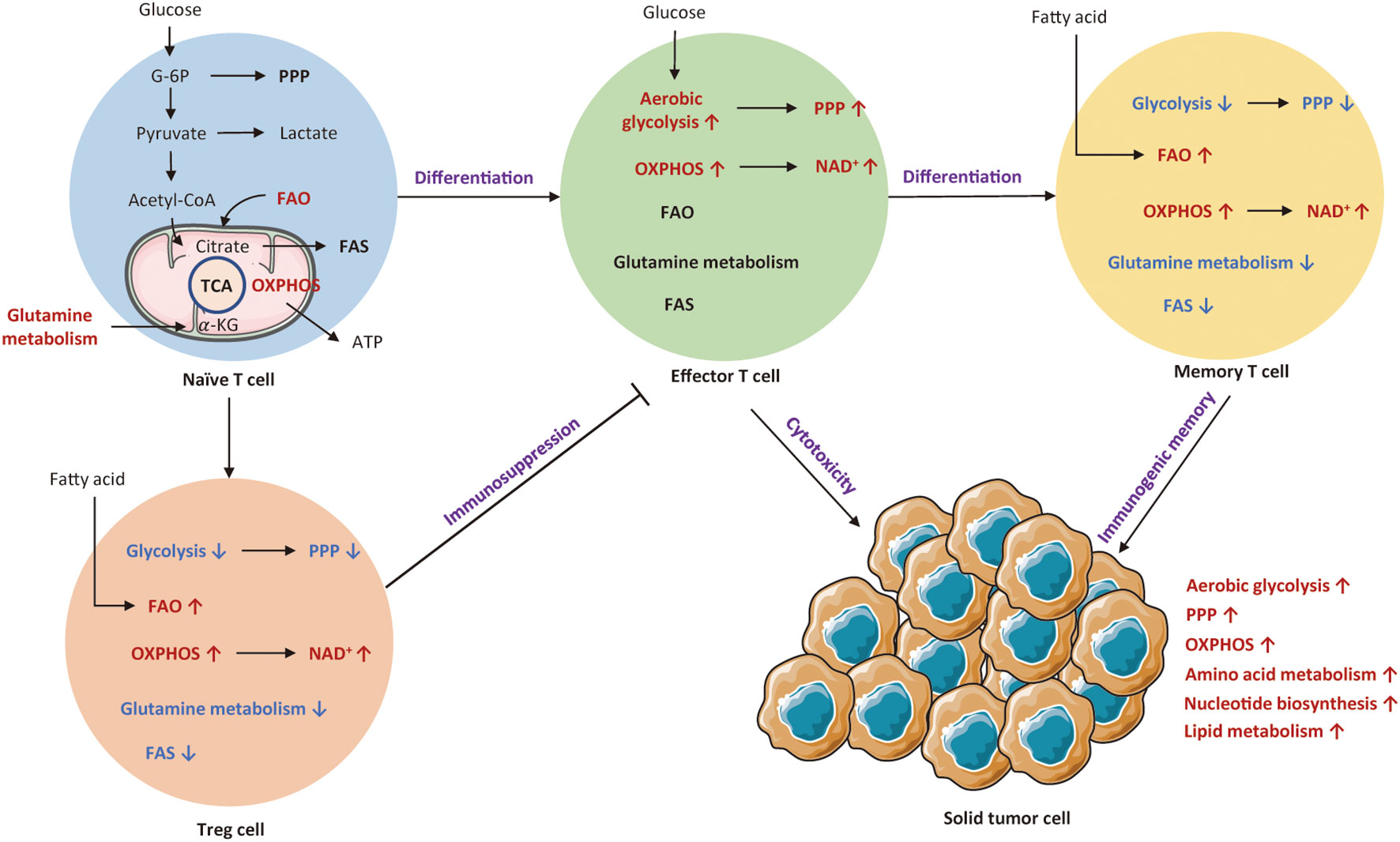
Abstract Tumour initiation and progression requires the metabolic reprogramming of cancer cells. Cancer cells autonomously alter their flux through various metabolic pathways in order to meet the increased bioenergetic and biosynthetic demand as well as mitigate oxidative stress required for cancer cell proliferation and survival. Cancer driver mutations coupled with environmental This review by Xiao et al. summarizes the metabolic targets for cancer treatment and provides an up-to-date overview of clinical trials targeting cancer metabolism. They decipher crucial factors that limit the effectiveness of metabolism-based therapies and suggest future directions to advance personalized metabolic therapies for better cancer treatment. In-depth research on the reprogramming of energy metabolism in T cells can reveal the mechanisms underlying tumor immune tolerance and provide important clues for the development of new tumor immunotherapy strategies as well. Therefore, the study of T cell energy metabolism has important clinical signi cance and fi potential applications.
Normal cells mainly rely on oxidative phosphorylation as an effective energy source in the presence of oxygen. In contrast, most cancer cells use less The abnormal metabolism of substance and energy in tumor cells is not only the cause of occurrence and development of malignant tumors, but also the concrete embodiment of their pathological changes. Emerging evidence indicates that metabolism not only is a source of energy and biomaterials for cell division but also acts as a driver of cancer cell plasticity and treatment resistance. This is because metabolic changes lead to remodeling of chromatin and reprogramming of gene expression patterns, furthering tumor cell phenotypic transitions.
The hallmarks of cancer metabolism: Still emerging
Lactate metabolism and lactylation in cells. In the cytoplasm, lactate is transported into cells by MCTs and is produced from glycolysis or glutamine decomposition. The catabolism of lactate in
After the discovery of based on the altered cancer cell metabolism in 1930, loads of studies have shed light on several aspects of cancer metabolism with a common goal to find new ways for effectively eliminating tumor cells by targeting their energy metabolism.
One process in leukemia stem cell biology that has intriguing therapeutic potential is energy metabolism. In this article we discuss the metabolic properties of leukemia stem cells and how targeting energy metabolism may provide more effective therapeutic regimens for leukemia patients. Cancer cells have a greater need for energy and a ready supply of the building blocks necessary for the synthesis of macromolecules (nucleotides, protein, lipids) in order to duplicate genome and biomass. The hypothesis can be postulated that those
Cancer cells have a greater need for energy and a ready supply of the building blocks necessary for the synthesis of macromolecules (nucleotides, protein, lipids) in order to duplicate genome and biomass. The hypothesis can be postulated that those precursors for synthetic processes, which can only be furnished by glycolysis, cannot be sufficiently recruited Abstract Tumor energy metabolism plays a crucial role in the occurrence, progression, and drug resistance of tumors. The study of tumor energy metabolism has gradually become an emerging field of tumor treatment. Recent studies have shown that epigenetic regulation is closely linked to tumor energy metabolism, influencing the metabolic remod-eling and biological traits of tumor
- Enfisema Mediastínico , Enfisema subcutáneo relacionado con trauma facial en un adulto
- Endloses Lesen Mitten In Koblenz Stadtlesen Auf Jubiläumstour
- Endnote X9 Now Available! _ EndNote链接pubmed报错怎么办?(亲测有效)
- English Translation Of ‚Bianco‘
- English Tenses Tutorial For Beginners
- Endomondo Apk Download – Endomondo Android Apl APK
- Energix Group Reviews: What Is It Like To Work At Energix Group?
- English Translation Of ‚Auszubildende‘
- Englische Lektionen Für Das Thema Essen Und Trinken Gratis Online
- Energieeffiziente Drehstrom-Asynchronmotoren
- Endothelial Healing In Vein Grafts
- England 2. Weltkrieg Tropenhelm
- English Translation Of ‚Nachschlagen‘