Discoidin Domain , Discoidin Domain Receptor
Di: Ava
The discoidin domain receptors (DDRs) are collagen-binding receptor tyrosine kinases that have been implicated in a number of fundamental biological processes ranging from growth and development to immunoregulation. In this review, we examine how recent proteomic technologies have enriched our understanding of DDR signaling mechanisms. We provide an Discoidin domain receptor 1 (DDR1) encodes a receptor tyrosine kinase involved in multiple physiological and pathological processes. DDR1 is expressed in the intestinal epithelium, but its role in Ulcerative Colitis (UC) is poorly understand. This study aimed to identify the function of DDR1 in maintaining the homeostasis of UC. Discoidin domain receptor 1 is a potential target correlated with tumor invasion and immune infiltration in gastric cancer

Discoidin domain receptor 1 (DDR1) has a significant role in crucial cell survival processes. Upon collagen binding, autophosphorylation of its kinase domain leads to downstream signaling pathways. Abnormal expression of DDR1 has been linked with inflammatory diseases, fibrosis, and cancer progression. DDR1 is now extensively explored as a potential therapeutic target Discoidin (DS) domains are found in eukaryotic and prokaryotic extracellular and transmembrane multidomain proteins. These small domains
Complete information for DDR2 gene (Protein Coding), Discoidin Domain Receptor Tyrosine Kinase 2, including: function, proteins, disorders, pathways, orthologs, and expression. GeneCards – The Human Gene Compendium The discoidin domain receptors (DDRs) are collagen-binding receptor tyrosine kinases that have been implicated in a number of fundamental biological processes ranging from growth and development to immunoregulation. In this review, we examine how
Discoidin domain receptors: Micro insights into macro assemblies
Discoidin domain receptors (DDR1 and DDR2) are the collagen receptors of the family tyrosine kinases, which play significant role in the diseases like inflammation, fibrosis and cancer. Chronic In this article, we review the recent published literature on the role of discoidin domain receptors (DDR) in cancer progression, the development status of DDR inhibitors, and the clinical potential 1. Introduction Discoidin domain receptors (DDRs) are widely expressed cell-surface receptors belonging to two major families of receptors. The first among these is the receptor tyrosine kinase (RTK) family. RTKs are cell-surface receptors that are characterized by a ligand-binding extracellular domain (ECD), a single transmembrane domain (TMD) and an
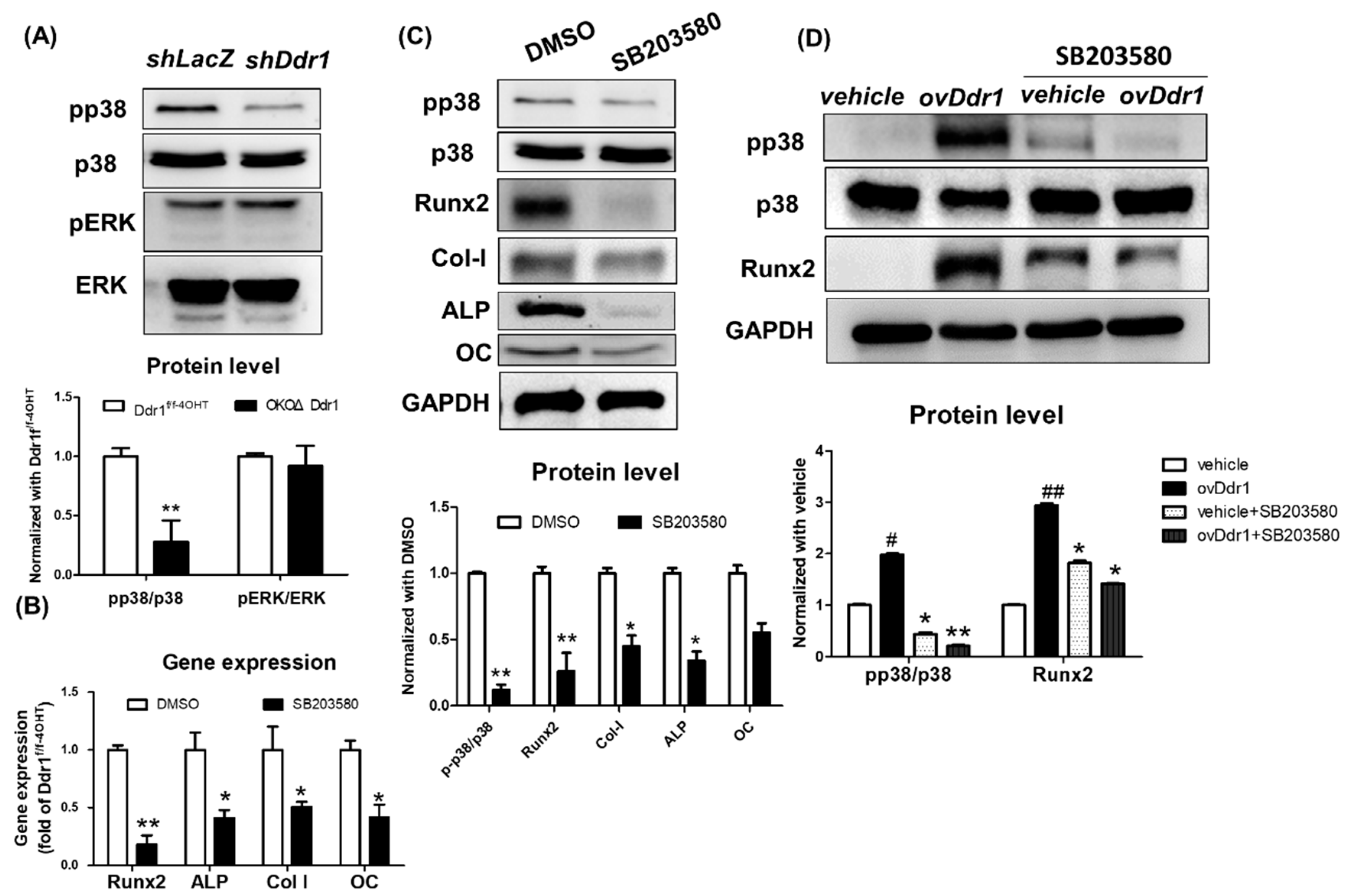
The discoidin domain receptors are a second less studied class of collagen receptors having important functions in bone development and regeneration (for review 17). The discoidin domain receptors, DDR1 and DDR2, are nonintegrin collagen receptors that are members of the receptor tyrosine kinase family. Both DDRs bind a number of different collagen types and play important roles in embryo development. Dysregulated DDR function is associated with progression of v
Skeletal progenitor: collagen interactions are critical for bone development and regeneration. Both collagen-binding integrins and discoidin domain receptors (DDR1 and DDR2) function as collagen receptors in bone. Each receptor is activated by a distinct collagen sequence; GFOGER for integrins and GVMGFO for DDRs. Specific triple helical peptides containing each DDR Inhibitor是一种高效的盘状结构域受体 (discoidin domain receptor) 抑制剂,对 DDR2 的IC50值为 3.3 nM,在浓度为 1.5 nM 时,对 DDR1 有 53% 的抑制作用。
- Discoidin domain receptors: a proteomic portrait
- Expression and mutation analysis of the discoidin domain
- The collagen receptor discoidin domain receptor 2 stabilizes
- DDR1 和 DDR2 双靶点抑制剂的设计合成及其抗炎作用研究
Discoidin domain receptor family, member 1, also known as DDR1 or CD167a (cluster of differentiation 167a), is a human gene. [5] Discoidin domain receptor 1 (DDR1) is a unique collagen-activated tyrosine kinase that participates in various human diseases, including cancer. DDR1 promotes adhesion, proliferation, differentiation, migration, and metastasis of cancer cells.
Discoidin domain receptor 1 (DDR1) has been reported to be negatively related to immune cell infiltration in tumors. Herein, we constructed a soluble fusion protein using CD80, the natural ligand of CD28, in combination with DDR1 inhibitor.
The discoidin domain is a ∼ 150 amino acid motif common in both eukaryotic and prokaryotic proteins. It is found in a variety of extracellular, intracellular and transmembrane multidomain proteins characterized by a considerable functional diversity, mostly involved in developmental processes. The biological role of the domain depends on its interactions with
Discoidin Domain Receptor
Discoidin domain receptor tyrosine kinases (DDRs) are a class of receptor tyrosine kinases (RTKs), and their dysregulation is associated with multiple diseases (including cancer, chronic inflammatory conditions, and fibrosis). The DDR family members
Discoidin domain receptor inhibitor DDR1-IN-1 induces autophagy and necroptotic cell death in malignant peripheral nerve sheath tumor Guan-Yi Lai, Yu-Cheng Lee, Hao-Jui Weng, Kuei-Hung Lai, Background The Discoidin Domain Receptor 1 (DDR1) is one of the two members of a unique family of receptor tyrosine kinase receptors that signal in response to collagen, which has been implicated in cancer progression. Here, we examined the expression of DDR1 in prostate cancer (PCa), and assessed its potential value as a prognostic marker, as a function Discoidin domain receptor 1 (DDR1) is a receptor tyrosine kinase that binds and is activated by collagens. Transcriptional profiling of cirrhosis in human liver using a DNA array and quantitative PCR detected elevated mRNA expression of DDR1 compared with that in nondiseased liver. The present study characterized DDR1 expression in cirrhotic and nondiseased human liver and
Type IV collagen plays a dual role in both structurally fastening tissues and signaling through the discoidin domain receptor 2 to synchronize an integrin adhesion that stabilizes tissue linkage. The onset and progression of tumors involve intricate, multifactorial processes. A key component in tumor evolution is the dynamic interaction between cancer cells and the extracellular matrix (ECM). Discoidin Domain Receptors (DDRs), a unique class of collagen-activated receptor tyrosine kinases, serve as critical mediators of cell-ECM communication. UniProt is the world’s leading high-quality, comprehensive and freely accessible resource of protein sequence and functional information.
Abstract Background: Discoidin domain receptor is a new and unique type of receptor tyrosine kinases, which binds to collagen, the main compose of an extracellular matrix. DDR1 was identified to mediate cell aggregation, and dysregulation of DDR2 has also been shown to be involved in tumor pathogenesis, although its role in cancer development and progression
The discoidin domain receptors (DDRs) are receptor tyrosine kinases that recognize collagens as their ligands. DDRs display unique structural features and distinctive activation kinetics, which set them apart from other members of the kinase superfamily. DDRs regulate cell-collagen interactions in normal and pathological conditions and thus are emerging Discoidin domain receptors (DDRs) are members of the transmembrane receptor tyrosine kinase (RTK) superfamily which are distinguished from others by the presence of a discoidin motif in the extracellular domain and their utilization of collagens as internal ligands. Two types of DDRs, DDR1 and DDR2, have been identified with distinct expression profiles and ligand specificities.
The tyrosine kinase family receptor of discoidin domain receptors (DDR1 and DDR2) is known to be activated by extracellular matrix collagen catalytic Complete information for DDR1 gene (Protein Coding), Discoidin Domain Receptor Tyrosine Kinase 1, including: function, proteins, disorders, pathways, orthologs, and expression. GeneCards – The Human Gene Compendium
Discoidin domain receptor 1 and DDR2 share similar structures, consisting of a characteristic discoidin homology domain, stalk region, transmembrane region, juxtamembrane region, and kinase domain. RS1, also known as retinoschisin, is an extracellular discoidin domain containing protein that has been implicated in maintaining the cellular organization and synaptic structure of the vertebrate retina. Mutations in the gene encoding RS1 are
Discoidin domain receptors (DDRs), including DDR1 and DDR2, are a unique class of receptor tyrosine kinases (RTKs) activated by collagens at the cell- UniProt is the world’s leading high-quality, comprehensive and freely accessible resource of protein sequence and functional information. The discoidin domain receptors (DDRs) are receptor tyrosine kinases that recognize collagens as their ligands. DDRs display unique structural features and distinctive activation kinetics, which set them apart from other members of the kinase
Two mammalian receptor tyrosine kinases (DDR1 and DDR2) have extracellular domains closely related to a D. discoideum lectin, discoidin, required for cell aggregation. Here, we show that the mammalian DDR receptors bind and are activated by specific types of collagen. Stimulation of DDR receptor tyrosine kinase activity requires the native triple-helical structure of collagen and
- Direct Transfers Vs. 60-Day Rollovers
- Discover What Causes “The Munchies” When You’Re High
- Direkt Wirkende Dampfmaschine : Dampfkessel- und Dampfmaschinen-Statistik.
- Directions To Corona, Ca | Directions to Trabuco Canyon, CA
- Diplomat Füllhalter Aero Blau Feder M
- Dirty Rap Classics On Tidal | Brazil Classics 1: Beleza Tropical by Various Artists on TIDAL
- Direktkandidat:Innen Zur Landtagswahl In Willich
- Discovery Whisky 70Cl : Yoichi Discovery Aromatic Yeast 2022 48% 70cl
- Directrices Para El Uso De Declaraciones Nutricionales
- Discovery Workshop „The Stellar Odyssey“
- Disco Duro Externo Portátil Elements De 2 Tb