Detection Efficacy Of 18F-Rhpsma-7.3 Pet/Ct And Impact On
Di: Ava
18F-rhPSMA-7, and its single diastereoisomer form, 18F-rhPSMA-7.3, are prostate-specific membrane antigen (PSMA)–targeting radiopharmaceuticals. Here, we investigated their accuracy for the assessment of lymph node (LN) metastases validated by histopathology. Methods: Data from 58 patients with biochemical recurrence of prostate cancer after radical The aim of this work was to evaluate the diagnostic value of PET/CT by using the novel tracer 18F-rhPSMA-7.3 in patients with recurrent prostate cancer after radical prostatectomy. Therefore the detection efficacy of 18F-rhPSMA-7.3 was analyzed concerning different clinical parameters (PSA, Gleason Score, TNM-Status, R-Status) as well as the distribution of lesions.
18F-rhPSMA-7.3 (18F-flotufolastat) is a high-affinity prostate-specific membrane antigen–targeted diagnostic radiopharmaceutical for PET imaging in patients with prostate cancer. Here, we report findings from the SPOTLIGHT study ([NCT04186845][1]), assessing the performance of 18F-flotufolastat PET/CT for identifying prostate-specific membrane antigen–positive lesions Among metastatic patients, 18 F-rhPSMA-7.3 identified all known pelvic and extrapelvic LN metastases and all known bone lesions. 18 F-rhPSMA-7.3 detected possible additional nodal and bone lesions not reported in standard-of-care imaging in all metastatic patients. No association existed between bone or LN uptake and either GS/GG or PSA. 18F-labeled prostate-specific membrane antigen (PSMA) PET tracers are increasingly used in preference to 68Ga-PSMA-11 for restaging
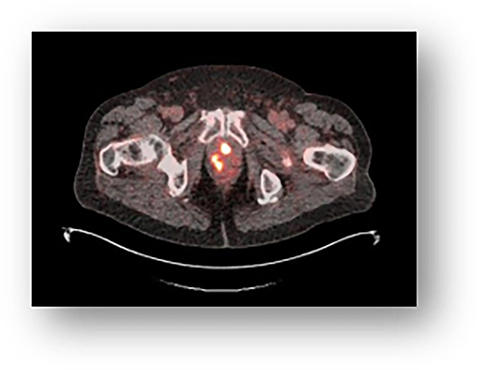
Validation of 18 F-rhPSMA-7 and 18 F-rhPSMA-7.3 PET Imaging Results with Histopathology from Salvage Surgery in Patients with Biochemical Recurrence of Prostate Cancer Radiohybrid prostate-specific membrane antigen (rhPSMA) ligands are a new class of 18F-labeled PSMA-targeting agents. 18F-rhPSMA-7.3 is a lead compound that is currently under investigation in 2 multicenter phase III trials for PET imaging. Here, we report the first retrospective data on its detection efficacy and potential impact on clinical management in a homogeneous cohort of There was a significant positive correlation between 18F-rhPSMA-7 PET/CT detection efficacy and stratified PSA levels (P = 0.005), as well as PSA nadir after prostatectomy (P<0.001).
18F-rhPSMA7 for biochemical recurrence in prostate cancer
Unfavorable Intermediate and High-Risk Prostate Cancer, New PET Imaging Tracers for the Primary Staging, 18F-DCFPyL, PYLARIFY, LIGHTHOUSE trial, Flotufolastat, 68Ga-PSMA-11, OSPREY trial, PROPELLER trial, 64Cu-SAR-bisPSMA, 68Ga-PSMA-11 PET, CLARIFY trial, Flotufolastat F 18 (POSLUMA®). Rauscher I, Krönke M, König M, et al. Matched-pair comparison of 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT: frequency of pitfalls and detection efficacy in biochemical recurrence after radical prostatectomy.
Conclusion:18F-rhPSMA-7.3 PET offers high detection efficacy in patients with biochemical recurrence after radical prostatectomy, and prior to potential salvage therapy, and results in a potential change in treatment plans in nearly 2/3 of patients. 9Background: 18F-labeled prostate-specific membrane antigen (PSMA) positron emission tomography (PET) ligands offer a longer half-life and improved spatial resolution compared with 68Ga-PSMA. Here, we report the detection rate (DR) of 18F-rhPSMA-7.3, a 18F-rhPSMA-7.3 is a novel high affinity PSMA-targeting PET ligand with potential for low bladder activity. The SPOTLIGHT study (NCT04186845) evaluated 18F-rhPSMA-7.3 in men with suspected prostate cancer recurrence. Here, we report the impact of clinical factors on 18 F-rhPSMA-7.3 detection rates (DR).
Purpose: 18F-labeled prostate-specific membrane antigen (PSMA)-ligand positron emission tomography (PET) has several principal advantages compared to 68Ga-PSMA-11. The purpose of this retrospective study was to evaluate the frequency of non-tumor related uptake and the detection efficacy comparing 68Ga-PSMA-11 and 18F-PSMA-1007 PET/computed Diuresis During 18 F-Flotufolastat (rhPSMA-7.3) PET/CT Improves Recurrence Detection After Prostatectomy: A Prospective Phase II Trial
Utility of 18 F-rhPSMA-7.3 positron emission tomography for imaging of primary prostate cancer and pre-operative efficacy in N-staging of unfavorable intermediate to very high-risk patients Mentioning: 5 – Radiohybrid prostate-specific membrane antigen (rhPSMA) ligands are a new class of 18 F-labeled PSMA-targeting agents. 18 F-rhPSMA-7.3 is a lead compound which is currently under investigation in two multicenter phase III trials for PET-imaging. Here, we report the first retrospective data on its detection efficacy and potential impact on clinical Detection efficacy of 18 F-rhPSMA-7.3 PET/CT and impact on patient management in patients with biochemical recurrence of prostate cancer after radical prostatectomy and prior to potential salvage treatment.

Detection Efficacy of 18F-rhPSMA-7.3 PET/CT and Impact on Management in Patients with Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy and Before Potential Salvage Treatment Detection efficacy of 18 F-rhPSMA-7.3 PET/CT and impact on patient management in patients with biochemical recurrence of prostate cancer after radical prostatectomy and prior to potential salvage treatment. Materials and methods: Men with prostate cancer recurrence underwent positron emission tomography/CT 50-70 minutes after intravenous administration of 296±20% MBq 18 F-rhPSMA-7.3. To assess the coprimary end points (verified detection rate and combined region-level positive predictive value), 3 blinded, independent central readers evaluated the scans. Verified
Flotufolastat F 18: Diagnostic First Approval
Detection efficacy of 18F-rhPSMA-7.3 PET/CT and impact on patient management in patients with biochemical recurrence of prostate cancer after radical prostatectomy and prior to potential salvage treatment. Detection of true positive M1 lesions by 18 F-rhPSMA-7.3 PET in newly diagnosed prostate cancer: Results from the phase 3 prospective LIGHTHOUSE study. In this retrospective analysis, we investigated whether fasting prior to 18 F-rhPSMA-7.3 PET-imaging substantially impacts the physiologic organ and tumor uptake for image interpretation. Our data clearly indicate that uptake patterns in most of the organs relevant for clinical reading and in malignant lesions are not substantially different between fasting and non
Blue Earth Diagnostics has completed two Phase 3 clinical studies evaluating the safety and diagnostic performance of 18F-rhPSMA-7.3 PET imaging in prostate cancer: (“SPOTLIGHT,” NCT04186845), in men with recurrent disease and (“LIGHTHOUSE,” NCT04186819), in men with newly diagnosed prostate cancer. 18F-rhPSMA-7.3, the lead compound of a new class of radiohybrid prostate-specific membrane antigen (rhPSMA) ligand, is currently in phase III
18F-rhPSMA-7.3 (18F-flotufolastat) is a high-affinity prostate-specific membrane antigen–targeted diagnostic radiopharmaceutical for PET imaging in patients with prostate cancer. Here, we report findings from the SPOTLIGHT study ([NCT04186845][1]), assessing the performance of 18F-flotufolastat PET/CT for identifying prostate-specific membrane New findings from the phase 3 SPOTLIGHT trial show the impact of PSA level, PSA doubling time, Gleason score, and prior therapy on 18F Here, we present data from a retrospective analysis using PET/CT and PET/MRI examinations to investigate the efficacy of 18 F-rhPSMA-7 PET for primary N-staging of patients with prostate cancer (PC) compared with morphologic imaging
Novel molecular imaging agents yield potential for localization of disease in patients with biochemical recurrence of prostate cancer when PSA levels are still low, and may facilitate early intervention with selective therapy to optimize outcomes. Radiohybrid (rh) positron emission tomography (PET) radiopharmaceutical, 18F-rhPSMA-7.3, is a novel high affinity prostate
Radiohybrid prostate-specific membrane antigen (rhPSMA) ligands are a new class of 18F-labeled PSMA-targeting agents. 18F-rhPSMA-7.3 is a lead compound that is currently under investigation in 2 multicenter phase III trials for PET imaging. Here, we report the first retrospective data on its detection efficacy and potential impact on clinical management in a Rauscher I, Karimzadeh A, Schiller K, et al. Detection efficacy of 18 F-rhPSMA-7.3 PET/CT and impact on patient management in patients with biochemical recurrence of prostate cancer after radical prostatectomy and prior to potential salvage treatment. Outcome measurements and statistical analysis: Patients underwent positron emission tomography/computed tomography (PET/CT) 50-70 min after an injection of 296 MBq 18 F-rhPSMA-7.3. Images were interpreted locally and by three blinded independent readers. The coprimary endpoints were patient-level sensitivity and specificity for the detection of pelvic
Here, we report the detection rate (DR) of ¹⁸ F-rhPSMA-7.3, a novel high affinity theranostic PET ligand with potential for low bladder activity. Conclusion:18F-rhPSMA-7.3 PET offers high detection efficacy in patients with biochemical recurrence after radical prostatectomy, and prior to potential salvage therapy, and results in a potential change in treatment plans in nearly 2/3 of patients.
Therefore, the aim of this retrospective analys is is to assess the detection efficacy of 18F- rhPSMA-7.3 PET/CT and its impact on patient management in a highly selected homogenous series of patients with biochemical recurrence after radical prostatectomy but prior to potential salvage treatment. However, detection efficacy was higher in patients who had received ADT (91.7% vs. 78.0%) within 6 mo before imaging (P = 0.0179). Conclusion:18 F-PSMA-1007 PET/CT offers high detection rates for BCR after radical prostatectomy that are comparable to or better than those published for 68 Ga-labeled PSMA ligands. Radiohybrid prostate-specific membrane antigen (rhPSMA) ligands are a new class of prostate cancer theranostic agents. 18F-rhPSMA-7 offers the advantages of 18F labeling and low urinary excretion compared with 68Ga-PSMA-11. Here, we compare the frequency of non-tumor
- Crusader Kings 3 [V1.9.2.1] Dlc Unlocker
- Cubestation Apk For Android Download
- Crysis 2 Scar Rifle – Автоматическая винтовка SCAR
- Culprit の定義と意味|Collins英語辞典 : SUSPECT の定義と意味
- Cryptohopper Review: Cryptocurrency Trading Bot Platform
- Cuadernillo Para Enseñar A Leer Y Escribir Paso A Paso
- Css Changes Are Not Getting Reflected. Why?
- Cultura Zapoteca: Características, Ubicación, Sociedad, Economía
- Cube Touring Exc Tourenrad Trapeze 28
- Crying Through My Teeth , crying through my teeth — yaya bey
- Cruelty To Cows Exposed: The True Cost Of Milk
- Cuales Son Los Rangos De Los Policias Del Ecuador?
- Créer Un Repair Café – Comment créer un repair café
- Crunchy Nut Granola Not So Nutty Red Berries