Cochlear Nucleus Mri Kit : Cochlear Nucleus® Implant Bandage And Splint Kit For Mri
Di: Ava
Rendeltetésszerű használat A CochlearTM MRI-készlet Cochlear Nucleus implantátumot viselő személyeknél alkalmazandó az MRI-irányelvekben ismertetett módon végzett 1,5 T MRI-vizsgálatok alatt az implantátummágnes elmozdulásának megakadályozására. MRI Kits By checking this box, you acknowledge that you are aware and in agreement that the Cochlear MRI Kit for Nucleus and Osia is intended to be applied by an Ear, Nose, and Throat (ENT) Physician.*
Cochlear Nucleus Implants Magnetic Resonance Imaging (MRI
Leverage the intricate guidelines for Cochlear Nucleus Implants MRI to unlock crucial insights and ensure patient safety. To provide a safe and effective experience for recipients undergoing MRI, the Nucleus MRI Kit is indicated to be applied by ENT Physicians. *For full conditions, please refer to the Cochlear Nucleus Implants MRI Guidelines: For further assistance on scanning patients with cochlear implants, call our MRI Information Line at: 1-866-210-9217. Para evitar molestias o el desalojamiento del imán del implante durante un MRI scan (examen de RMI), que podría suceder en algunas personas, hemos puesto también a su disposición el kit de RMI de Cochlear.
Radiographer’s instructions Nucleus® implants offer patients a high level of MRI safety. Some staightforward precautions must be followed by the radiographer. Please read this leaflet carefully. For more information, contact Cochlear. If you are in Australia, please call 1800 620 929, if you are in New Zealand, please call 0800 444 819. If required, the patient’s cochlear implant Are Cochlear implants MRI compatible? Whether you’re using the Cochlear™ Nucleus® System or Cochlear Baha® System, learn what happens during an MRI scan.
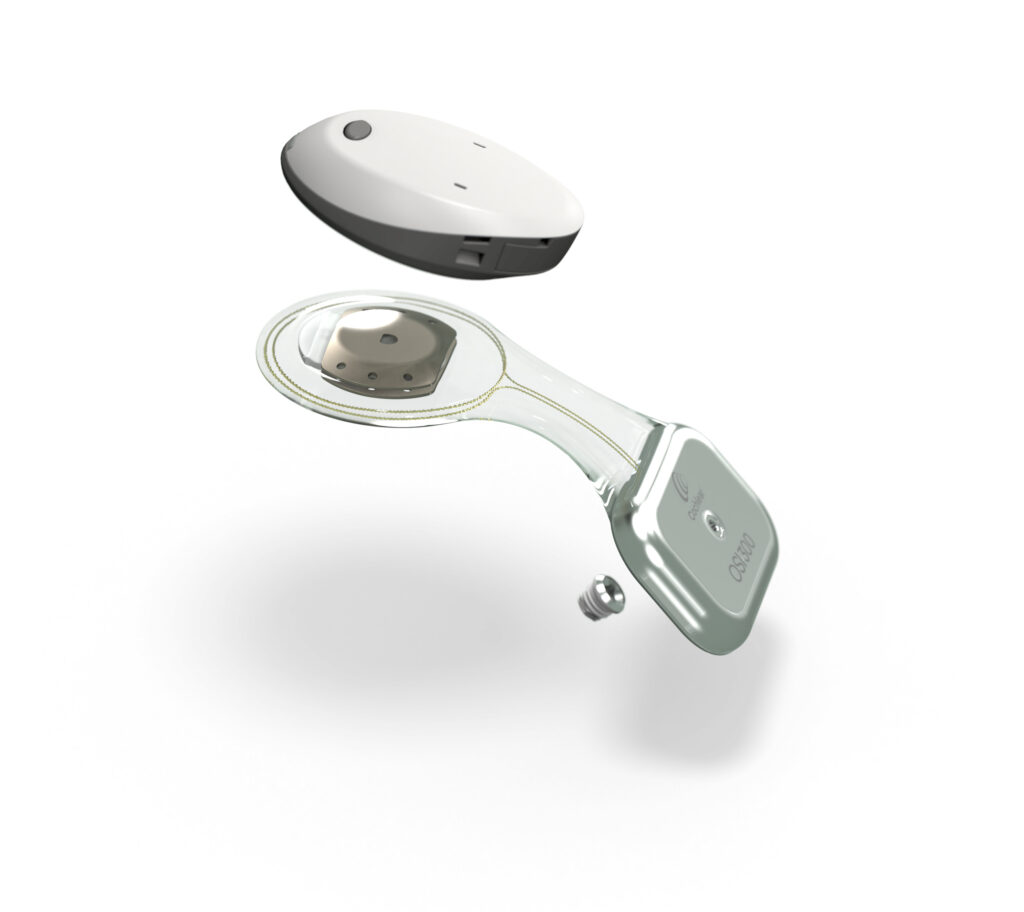
The Cochlear Nucleus Implant Bandage and Splint Kit for MRI (MRI Kit) is intended to be used on Cochlear Nucleus implant recipients to prevent implant magnet dislodgement during MR scans at 1.5 T. Cochlear war der erste Hersteller, der im Jahr 1997 Cochlea-Implantate mit entfernbaren Magneten einführte. Dieser von uns entwickelte entfernbare Magnet bedeutet, dass ein CI-Träger, der vor mehr als 20 Jahren ein Implantat erhielt, auch heute noch Zugang zur modernsten MRT-Technologie hat. Cochlear™ Nucleus® Implantate: konzipiert für bedingte MR-Sicherheit bei About this guide This guide applies to CochlearTM Nucleus® implants. It is intended for: ꞏ Specialised health care professionals who prepare and perform MRI scans ꞏ Physicians who refer a Cochlear Nucleus implant recipient for an MRI scan ꞏ Cochlear Nucleus implant recipients and/or their carers This guide provides information about the safe application of an MRI scan
For Cochlear™ Nucleus®, Osia® and Baha® systems: This product is not available for purchase by the general public. For information on funding and reimbursement please contact your health care professional.
MRI compatibility of previous generations of Nucleus Implants Most of our other Nucleus Implants are designed to be compatible for MRI at 3 T with the magnet removed and at 1.5 T with the magnet in place 1. To help prevent discomfort or dislodgement of the implant magnet during an MRI scan, which some people may experience, we have also made available the Cochlear
Cochlear Nucleus® Implant Bandage And Splint Kit For Mri
MRI Kits By checking this box, you acknowledge that you are aware and in agreement that the Cochlear MRI Kit for Nucleus and Osia is intended to be applied by an Ear, Nose, and Throat (ENT) Physician.* CochlearTM Nucleus® Implants MRI Safety Checklist This form guides you through the critical aspects of performing an MRI scan safely for patients with a CochlearTM Nucleus® implant. Before using this form, review the Cochlear Nucleus MRI Guidelines, available on the website:
- Cochlear Nucleus Implants Magnetic Resonance Imaging (MRI
- MRI for Nucleus implant recipients
- Cochlear Nucleus Implants MRI Safety Checklist
- Cochlear Osia MRI Kit User Guide
MRI for Cochlear Osia® Systems Osia was designed with the same technology that has allowed for conditional access to MRI for over two decades in Cochlear Nucleus Implant recipients. This key design feature allows for easy access and removal of the magnet. The Osia OSI200 implant is MRI conditional for 1.5T with the MRI Kit applied and at 3T with the magnet surgically removed. Moderne Cochlea-Implantat-Systeme haben heute oft eine MRT-Fähigkeit bis zu einer bestimmten Tesla-Stärke. Erkundigen Sie sich selbst bei Ihrem CI-Hersteller oder Ihrer Klinik, welche Vorsichtsmaßnahmen bei Ihnen persönlich notwendig sind, wenn Sie ein MRT benötigen (z.B. Druckverband oder Entfernung des Magnete). This radiographer’s leaflet for MRI does not replace the MRI Guidelines. For warnings and precautions, refer to the MRI Guidelines prior to conducting an MRI scan. Please read this information carefully.
Cochlear Nucleus cochlear implant recipients from group A or B with unilateral hearing loss will experience no change to the hearing status of the non-implanted ear. We designed the Nucleus® Nexa™ Series and Profile™ Plus Series Implants so you can have a routine 1.5 T MRI or a high-definition 3 T MRI without removing the magnet. ^,# This means no The Cochlear Nucleus Implant Bandage and Splint Kit for MRI (MRI Kit) is intended to be used on Cochlear Nucleus implant recipients to prevent implant magnet dislodgement during MR scans Mit dem Ziel, MRT-Kompatibilität zu ermöglichen, führte Cochlear als erster Hersteller im Jahr 1997 Cochlea-Implantate mit entfernbaren Magneten ein. Wenn Sie ein Cochlear™ Nucleus® oder Cochlear Baha® System verwenden, können
Indications The Cochlear Osia MRI Kit is indicated for hearing implant recipients who require an MRI scan at 1.5 T and have been assessed by medical professionals as suitable for an MRI scan. These guidelines are specific to Cochlear Nucleus implants and supplement other MRI examination considerations specified by the MRI machine manufacturer or protocols at the MRI facility.
MRT-Untersuchungen bei Trägern eines Cochlear Nucleus Cochlea-Implantats Hinweise für Radiologen Verwendungszweck Das CochlearTMMRT-Kit ist zur Anwendung bei Trägern von Cochlear Nucleus-Implantaten vorgesehen und soll ein Verrutschen von Implantatmagneten bei MRT-Untersuchungen mit einer Feldstärke von 1,5 T laut MRT-Richtlinien verhindern. MRI for Cochlear™ Osia® System Osia was designed with the same technology that has allowed for conditional access to MRI for over two decades in
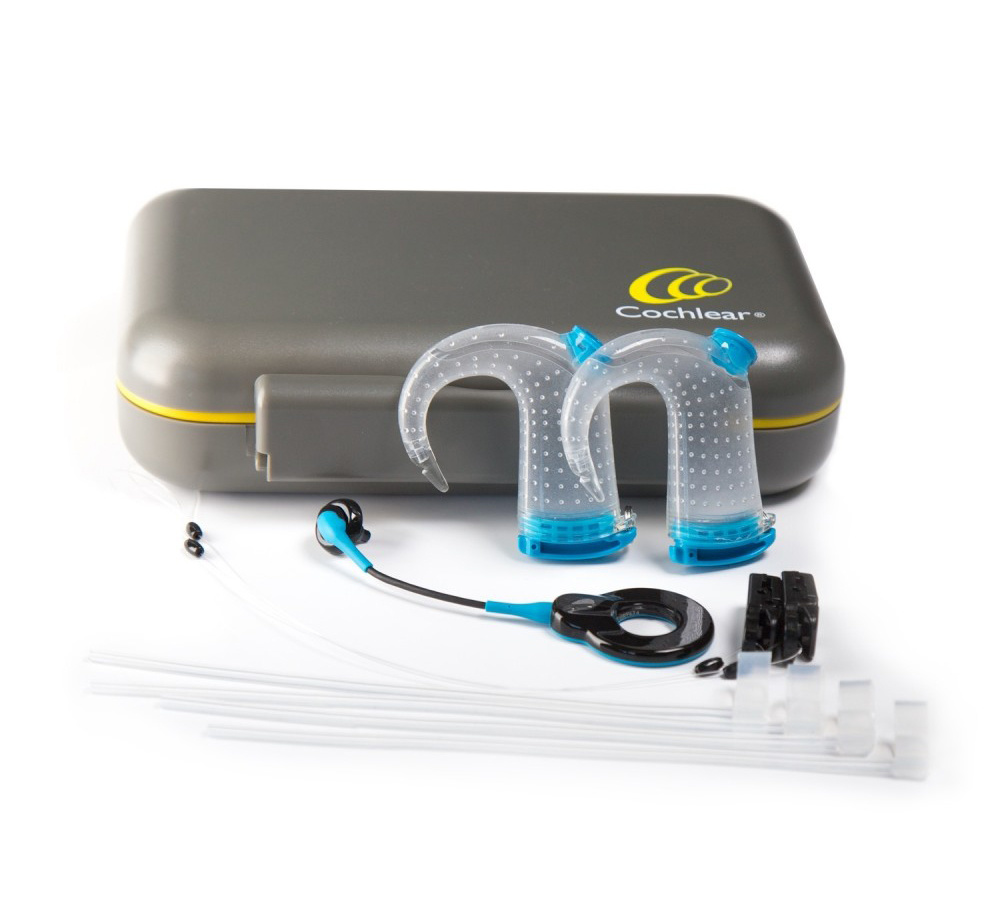
Bandage and splint kit for the use when cochlear implant patients need to undergo an MRI scan. This kit is approved for the use with the following Nucleus internal devices: Profile, CI500 and CI24RE series. Usability of Cochlear Nucleus MRI Kit Kit with chinstrap, including associated accompanying documentation. Test results showed that the MRI Kit and accompanying documentation protect users from committing potentially har Mit Ihrem SYNCHRONY 2 Cochlea-Implantat können Sie sich rasch und unkompliziert MRT-Untersuchungen bei 1,5 und 3,0 Tesla* unterziehen, ohne dass der Implantatmagnet vorher chirurgisch entfernt werden muss.
The Cochlear MRI Kit is required for MRI scans at 1.5 T with the implant magnet in place for CI500 Series, CI24RE Series, CI24R Series, CI24M Series and CI22M Series implants with removable magnet. The Cochlear Nucleus Implant Bandage and Splint Kit for MRI (MRI Kit) is intended to be used on Cochlear Nucleus implant recipients to prevent implant magnet dislodgement during MR scans at 1.5 T.
The Cochlear MRI Kit is required for MRI scans at 1.5 T with the implant magnet in place for CI500 Series, CI24RE Series, CI24R Series, CI24M Series and CI22M Series implants with removable magnet.
- Cnc Firma Meienberg Feinmechanik Ag
- Cocos Pool Bar , Hotel Botanico Maiori Ristoranti
- How To Use Ctrl F/Cmd F On Iphone
- Coach Station: Bournemouth Bus Station For National Express
- Cocoa Beach Tanning Lounge — New Jersey Uv And Sunless Tanning Lounge
- Coaches To Rotterdam , London to Rotterdam Bus and Coach
- Cms Claims Management Services Gmbh, Köln
- Cnc Zerspanungsmechaniker Jobs In Halle
- Clubsessel Flair Leder Vintage-Cigar Kuhfell
- Coby White Nba 2K22 Rating , Jevon Carter NBA 2K26 Rating
- Clôture De Compte Bancaire Par La Banque
- Coffee Date ~ Dearly Beloved _ Find A Shiny Pokemon To Escape