Chemical Bonding And Lewis Symbols
Di: Ava
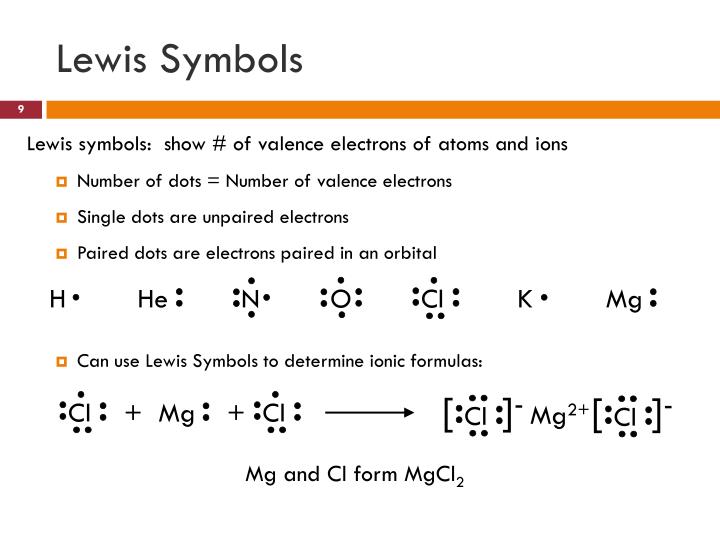
8.1: Lewis Symbols Lewis dot symbols can be used to predict the number of bonds formed by most elements in their compounds. Lewis electron dot symbols, which consist of the chemical symbol for an element surrounded by dots that represent its valence electrons, grouped into pairs often placed above, below, and to the left and right of the symbol. The structures reflect the fact Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond. Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol.
When atoms interact to form a chemical bond, only their outer regions are in con-tact. For this reason, when we study chemical bonding, we are concerned primarily with the valence electrons of the atoms. To keep track of valence electrons in a chem-ical reaction, and to make sure that the total number of electrons does not change, chemists use a system of dots devised by
Discover Gilbert N. Lewis’s model detailing chemical bond formation through Lewis Symbols and the Octet Rule. Understand bond types, electron configuration, exceptions of different elements, and how atoms decrease their potential energy
Chemical Bonding and Molecular Structure
Thus far, we have discussed the various types of bonds that form between atoms and/or ions. In all cases, these bonds involve the sharing or transfer of valence The document provides an overview of chemical bonding, including definitions of key terms such as chemical bonds, valence electrons, and Lewis symbols. It discusses different types of bonds (ionic, covalent, coordinate covalent, hydrogen, and metallic), their characteristics, and examples of compounds formed through these bonds. Additionally, it covers concepts like the octet rule, Thus far, we have discussed the various types of bonds that form between atoms and/or ions. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures.
Bonding and Lewis Symbols Bonds • Bonds are forces that hold groups of atoms together, making them function as a single unit • Bonds form when the energy of the two atoms together as a unit is lower than the separated atoms • Bond energy – energy required to break a chemical bond • Bonds can be largely, but not always entirely, understood in terms of electrostatic interactions In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. Lewis Symbols We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. 9.2 : Lewis Symbols and the Octet Rule Chemical bonds are complex interactions between two or more atoms or ions, which reduce the potential energy of the molecule. Gilbert N. Lewis developed a
CBSE Class 11 Chemistry Notes For Chapter 4 Chemical Bonding And Molecular Structure Theory Of Valency Kossel-Lewis Theory Earlier the term ‘valency’ was defined as the combining capacity of an element. In order words, an element can combine with another element. Theories of valency were a direct consequence of the development of the atomic structure. w.
Electrons that are not in the valence level are not shown in the Lewis symbol. The reason for this is that the chemical reactivity of an atom of the element is solely In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures.
chemical bonding edited pdf
- Chemical Bonding Basics: Lewis Symbols & Octet Rule
- Lewis Dot Structure: Definition, Examples, and Drawing
- Chemical Bonding and Molecular Structure
- Lewis Symbols and Structures
Lewis dot symbols can be used to predict the number of bonds formed by most elements in their compounds. Lewis electron dot symbols, which consist of the chemical symbol for an element surrounded by Lewis Symbols and the Octet Rule Lewis symbols are a fundamental tool in understanding chemical bonding. They depict elements with their valence electrons as dots placed around the element’s symbol, providing a visual representation of electron pairs involved in bonding. This method simplifies the process of tracking electrons during bond formation and helps predict
In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures.
Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures.
Lewis symbols, also known as electron dot diagrams, are a graphical representation of the valence electron configuration of an atom or molecule. They depict the arrangement of valence electrons around the nucleus of an atom, which is crucial for Lewis Theory of Chemical Bonding The Lewis structure derives its name from Gilbert N. Lewis, who developed the concept in 1916. Irving Langmuir introduced the idea in 1920, naming it and giving credit to Lewis. 66.4K Views. Chemical bonds are complex interactions between two or more atoms or ions, which reduce the potential energy of the molecule. Gilbert N. Lewis developed a model called the Lewis model that simplified the depiction of chemical bond formation and provided straightforward explanations for the chemical bonds seen in most common compounds. Lewis Model The
7.3 Lewis Symbols and Structures
Chemical Bonding and Molecular Structure Matter is composed of elements, with atoms typically forming molecules held together by chemical bonds. These bonds arise from attractive forces between atoms. Various theories, including Kössel-Lewis, VSEPR, VBT, and MOT, explain why atoms combine and how molecules form. Ultimately, chemical bonding is nature’s way of Lewis Diagrams for Compound Formation
Lewis Symbols: In the formation of a molecule, only the outer shell electrons take part in chemical combination and they are known as valence electrons. The inner shell electrons are well protected and are generally not involved in the combination process.
Lewis structure definition | What is a Lewis dot diagram? The formation of a chemical bond (ionic and covalent) involves the transfer of electrons or the exchange of electrons. Lewis structures can be used to represent valence shell electrons in a chemical bond. The Lewis structure, proposed by Gilbert Newton Lewis, who introduced it for the first time in 1916, is a graphic representation Lewis Symbols At the beginning of the 20 th century, the American chemist G. N. Lewis (1875–1946) devised a system of symbols—now called Lewis electron dot symbols (often shortened to Lewis dot symbols) that can be used for predicting the number of bonds formed by most elements in their compounds. Each Lewis dot symbol consists of the chemical symbol for
In this Chemistry video in Hindi for class 11 we explained Kossel – Lewis approach to chemical bonding. They came to the conclusion, independently, that every atom has tendency to attain the
Chemical bonding and molecular structure grade 11
Thus far, we have discussed the various types of bonds that form between atoms and/or ions. In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. How to Draw Electron Dot Structures? A Lewis Electron Dot Formula comprises one dot for every valence electron and an element’s symbol. Stages to articulate the electron dot formula are stated beneath. Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. Pick up every valence electrons from every atom and toss them into a
Objectives By the end of this section, you will be able to: Write Lewis symbols for neutral atoms and ions Draw Lewis structures depicting the bonding in simple Lewis dot symbols show the number of valence electrons and therefore, they are the basis of understanding the principles of chemical bonding which originate What is Lewis Structure Lewis structure, also known as Lewis dot structure or electron dot structure, is a simple and straightforward way of representing the outermost electron shell in a chemical species like an atom, ion, or molecule. It shows how electrons are positioned around the atoms either as lone pairs or in a chemical bond, typically a covalent bond or a coordinate
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting
Lewis Symbols At the beginning of the 20 th century, the American chemist G. N. Lewis (1875–1946) devised a system of symbols—now called Lewis electron dot symbols (often shortened to Lewis dot symbols) that can be used for predicting the number of bonds formed by most elements in their compounds. Each Lewis dot symbol consists of the chemical symbol for
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence electrons and chemical bonds, namely Lewis symbols and Lewis structures.
Chemical Bonds, Lewis Symbols, and the Octet Rule The properties of many materials can be understood in terms of their microscopic properties.
- Chicken Fontinella | 10 Best Fontina Cheese Recipes No One Can Resist
- Chi È Sia, La Cantante Di Chandelier E Titanium
- Cheeracademy.De Jetzt Kaufen! – "The Book That Will Cheer You" online kaufen
- Cheap Flights From Manchester To Zayed International
- Cheap Flights From Pereira To Medellin
- Check Out The Kratos Voice Ai That I Made!
- Cheerleader Jewelry _ Amazon.com: Cheerleader Charm
- Chf 150- Flüge Nach Siebenbürgen
- Cheap Flights From Sao Paulo To Lima, Peru
- Cheap Flights From Ulaanbaatar To Bangkok Suvarnabhumi
- Chemotaxis, Signal Relaying And Aggregation Morphology
- Chi Pads Mandarinen-Baumessig Fußreflexzonen-Pads, 10 Pcs.
- Cheap Wedding Dresses Online _ 15 Places To Buy Inexpensive Wedding Dresses
- Chiemgauhof In Feldwies Vor Dem Abriss
- Cheap Refurbished Dell All-In-One Desktop Computer Deals