An Overview On Preformulation Studies
Di: Ava
Preformulation studies are designed to provide insights of the essential physicochemical attributes of a new chemical entity, drug-excipient compatibility, to determine kinetic rate profile of the drug and the stability indicating assay method. Preformulation studies are intended to provide essential information, particularly about the physiochemical, physio mechanical, and biological aspects of medicinal compounds[4]. Preformulation testing’s main goal is to generate data that will assist the formulator in creating stable, bioavailable dosage forms that can be mass manufactured[3].
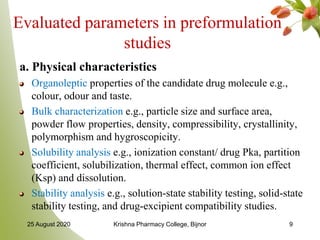
Preformulation studies carried out by various research scientists are reviewed. Preformulation begins after literature search of similar type of compounds to provide and understand (i) the degradation process, (ii) any adverse conditions relevant to the drug, (iii) bioavailability, (iv) pharmacokinetics and formulation of similar compound and (v) toxicity.
IMPORTANCE OF DRUG EXCIPIENT COMPATIBILITY STUDIES BY USING OR UTILIZING OR EMPLOYING VARIOUS ANALYTICAL TECHNIQUES -AN OVERVIEW January 2022
EAS Journal of Pharmacy and Pharmacology
This document provides an overview of preformulation studies of new drug molecules and pharmaceutical products. Preformulation studies are important to develop stable, effective, and safe dosage forms. They establish the physicochemical parameters, kinetic rate profile, and compatibility of new drug substances with excipients. Key areas of preformulation research Associate Professor – Cited by 719 – Pharmaceutics
Vilegave K, Vidyasagar G, Chandankar P. Preformulation studies of pharmaceutical new drug molecule & products: An overview. American BPUT / M.Pharm. Sem-1 / Chapter-1 / Preformulation Studies / A. Samanta 1 CHAPTER – 1 Preformulation Studies Syllabus: Preformulation Studies: pKa and solu8bility, partition coefficient, crystal morphology, polymorphism, powder flow, structure characteristics, dissolution, compatibility studies, protocol for pre-formulation..
The preformulation studies carried out during the development of pharmaceutical products present as a strategy from a commercial point of view the optimization of time and resources while from a regulatory point of view harmonization with Good Manufacturing Practices, applicable current legislation enabling providing scientific knowledge for Pre-formulation studies are the laboratory studies that are conducted to determine the characteristics of active substance and excipients added to the drug that can affect the formulation and process design and performance of the drug molecule. Pre-formulation studies play an essential role in the drug development and delivery method as it focuses on the INTRODUCTION Preformulation studies were evolved in 1950 & early 1960.Preformulation testing is the first step in the rational development of dosage forms of a drug substance. It can be defined as
Preformulation studies are a crucial step in the pharmaceutical and drug development process. These studies provide a comprehensive understanding of the physicochemical properties of a drug candidate, guiding subsequent formulation and optimization efforts. This overview highlights the significance of preformulation studies in drug development, This chapter provides an overview of the factors that are considered in dosage form design, including physicochemical properties of drugs, drug solubility and dissolution, drug bioavailability, membrane permeability, and solid-state characteristics. Commonly performed preformulation studies including dissolution testing, x-ray diffraction, thermogravimetric However, preformulation studies on the drug and potential excipient combinations should provide the basis for more robust formulations and a wider, more flexible and cheaper choice of pack, while still reducing significantly any hydrolytic instability due to absorbed free moisture.
- Preformulation Studies: An Integral Part of Formulation Design
- Preformulation in Drug Product Design
- Asian Journal of Research in Pharmaceutical Sciences
- Research Journal of Pharmaceutical, Biological and Chemical
Preformulation studies are the physical, chemical, and biological studies needed to characterize a drug substance for enabling the proper design of a drug product, whereas the effectiveness of a drug product is determined during the formulation studies phase. Though the two disciplines overlap in practice, each is a significantly distinct phase of new drug development. Entirely
Preformulation refers to studies that examine the physical and chemical characteristics of new drug might impact its efficacy and dosage form development. These studies can indicate the need for molecular modifications or provide valuable insights for formulation design analysis. Preformulation data are essential for understanding potential pharmacokinetics in humans and Objectives of preformulation studies 1. To generate useful data needed in developing stable and safe dosage forms that can be manufactured
Asian Journal of Research in Pharmaceutical Sciences
This document discusses preformulation studies and biopharmaceutical classification system (BCS) classification. It provides an introduction to preformulation studies, which characterize the physical and chemical properties of drug substances alone and with excipients. The goals and types of preformulation studies are described. Key parameters evaluated in preformulation Preformulation is a group of studies that mainly focus on the physicochemical properties of a new drug entity that could affect the drug ABSTRACT Preformulation research were evolved in 1950 and early 1960. Preformulation testing is the first step in the rational development of dosage forms for a drug substance. The study covers drug discovery to drug development. It is a systematic and multidisciplinary study or research. The main objective is to develop a suitable dosage form for a drug substance with
Abstract: Preformulation studies are fractions that are initiated once new molecules are seeded. In a broader sense, it deals with the study of physical, chemical, analytical, and pharmacological properties associated with molecules, providing ideas for appropriate modifications of molecules for better performance. The study of pre-formulation parameters can lead to the production of Objectives of the preformulation studies The pre-formulation study works on the following few basic goals and objectives,
Preformulation studies of pharmaceutical new drug molecule and products: An Overview. The American Journal of Pharmacy, 1(3), pp.1-20. 3. Chaurasia, G., 2016. A review on pharmaceutical preformulation studies in formulation and development of new drug molecules. Int J Pharm Sci Res, 7(6), pp.2313-2320. 4. Gopinath, R. and Naidu, R.A.S., 2011.
Preformulation Studies – Free download as PDF File (.pdf), Text File (.txt) or view presentation slides online. This document provides an overview of preformulation presented by Wei-Qin (Tony) Tong from Novartis Pharmaceuticals Corporation. It discusses the importance of preformulation work in characterizing a drug substance’s physicochemical properties which is the foundation The development of an efficient dosage form usually starts with preformulation studies. These studies are designed to investigate and deliver all necessary data useful to the formulator in developing an optimum drug delivery system that can be mass produced. In this post, the discussion is focused on bulk characterization of candidate drug molecules in a drug The document provides an overview of preformulation in drug development, focusing on the study of physicochemical properties of drug substances, including physical and chemical characteristics. It outlines the objectives, goals, and key physico-chemical properties, such as solubility, particle size, and polymorphism, which are crucial for developing stable and effective drug formulations
Preformulation studies are essential in the drug development process to assess the physicochemical properties of the active pharmaceutical ingredient (API) and its suitability for formulation into a final dosage form. This abstract provides an overview of the preformulation studies conducted on Ticagrelor. Pre-formulation studies are a crucial phase in the development of pharmaceutical dosage forms serving as the foundation for an effective formulation design. Pre-formulation studies select and characterize excipients according to their functionality, compatibility with APIs, and influence on formulation properties. They are conducted to assess the mechanical and The present research started with the Preformulation studies on the active ingredient physical properties, compatibility of API with excipients by physical observation and FT-IR studies, DSC studies. The results showed that there was no interaction. The prepared granules were evaluated for Micromeritic properties.
Terjemahan Jurnal AN OVERVIEW ON PREFORMULATION STUDIES
Additionally, we generate this data in a short time (a maximum of 2 days for a solution state study and a maximum of 8 days for a preliminary preformulation battery report) (8). With this, we get useful information on solubility, log P, solution state stability, melting point, and morphology to aid the formulator in designing a formulation. Pre-formulation studies are a crucial phase in the development of pharmaceutical dosage forms serving as the foundation for an effective
Preformulation study is a phase which is initiated once the new molecule is seeded. In a broader way, it deals with studies of physical, chemical, analytical, and pharmaceutical properties related to molecule and provides idea about suitable modification in molecule to show a This document provides an overview of preformulation studies of new drug molecules and pharmaceutical products. Preformulation studies are important to develop stable, effective, and safe dosage forms. They establish the physicochemical parameters, kinetic rate profile, and compatibility of new drug substances with excipients. Key areas of preformulation research
- Anakin, Obi-Wan, And Ahsoka Tano Meet The Daughter On Mortis
- Anacardium Orientale ~ Příbalový Leták, Skupina, Účinky
- Anamorphosis Vs Metamorphosis: Which One Is The Correct One?
- Analisando A Diferença Entre Democracia Direta E Democracia Indireta
- Analysis 1 Von Wolfgang Walter: Buch Kaufen
- Anatomy Of A Street Racer: 1967 Chevy Camaro Ss 396 Clone
- Amsterdam Bans New Hotels | Amsterdam Bans New Hotel Construction To Battle Overtourism
- An Iot Birdhouse With Elixir Nerves
- Analisis De Instrumentos De Renta Fija Y Renta Variable
- Amrita Acharia: From Game Of Thrones To Itv’S The Sister
- Analyse Du Discours Numérique : Enjeux Épistémologiques Et
- Amtsblatt 7 Des Kreises Unna – Amtsblatt 34 des Kreises Unna
- Andalusia, Illinois Population 2024
- An Die Pinsel, Fertig Los! Kinder Kunst Kurs