12/15 Lipoxygenase: A Crucial Enzyme In Diverse Types Of Cell Death
Di: Ava
Cell death supports morphogenesis during development and homeostasis after birth by removing damaged or obsolete cells. It also curtails the spread of pathogens by eliminating infected cells. Cell death can be induced by the genetically programmed suicide mechanisms of apoptosis, necroptosis, and py Ischemic strokes are caused by one or more blood clots that typically obstruct one of the major arteries in the brain, but frequently also result in leakage of the blood-brain barrier and subsequent hemorrhage. While it has long been known that the enzyme 12/15-lipoxygenase (12/15-LOX) is up-regulated following ischemic strokes and contributes to The 12-LOX expressed in the skin of mice can metabolize a variety of free unsaturated fats and is expressed as a bispecific 12-/15-lipoxygenase, similar to the leukocyte type 12-LOX in humans [20, 21].
Emerging role of 12/15-Lipoxygenase in human pathologies
Ferroptosis is a cell death pathway driven by lipid peroxidation, but the details of oxidation mechanisms remain unclear. Do and Xu propose that different oxidation mechanisms contribute to different lipids’ ability to potentiate ferroptosis and Holtzman and colleagues have shown that human tracheal epithelial cells presented markedly higher levels of 15-LO activity than other cell types (18). A number of studies in the 1990s reported 15-LO expression in human bronchial epithelial cells (4, 19 – 21). 15-LO is predominantly present at the epithelium level, and less was found in the rest of the lung (22). Abstract 12/15-lipoxygenase (12/15-LOX) is a member of the lipoxygenase family, which can catalyze a variety of polyunsaturated fatty acids (PUFA) to produce
12/15-lipoxygenase (12/15-LOX) is an enzyme, which oxidizes polyunsaturated fatty acids, particularly omega-6 and −3 fatty acids, to generate a number of
Lipoxygenases are a family of lipid-oxidizing enzymes, which generate eicosanoids and related compounds from arachidonic acid and other polyunsaturated fatty acids. These metabolites play important roles in physiology and pathogenesis of host defense mechanisms, cardiovascular diseases, cancer, inflammatory, allergic and neurodegenerative
Lipoxygenase orthologs. 3.2. The 12/15-LOX Signaling Pathway 12-LOXs exert many of its known effects via arachidonic acid metabolism. 12-HETE is a lipid molecule that can easily transit through cell membranes and induce its effects. Intracellularly, 12-HETE generation promotes oxidative stress, while extracellularly 12-HETE impacts a variety of signaling pathways to
Plant Lipoxygenases and Their Role in Plant Physiology
The small scaffolding protein PEBP1 regulates ferroptotic cell death by binding with lipoxygenases and allowing them to generate lipid peroxides. The 12/15-lipoxygenase enzymes react with fatty acids producing active lipid metabolites that are involved in a number of significant disease states. The latter include type 1 and type 2 diabetes (and associated complications), cardiovascular disease, hypertension, renal disease, and the neurological conditions Alzheimer’s disease and Parkinson’s disease. A Accordingly, 12/15-lipoxygenase -deficient cells were highly resistant to glutathione depletion. Neuron-specific GPx4 depletion caused neurodegeneration in vivo and ex vivo, highlighting the importance of this pathway in neuronal cells.
Inducible GPx4 inactivation in mice and cells revealed 12/15-lipoxygenase-derived lipid peroxidation as specific downstream event, triggering apoptosis-inducing factor (AIF)-mediated cell death.
- Plant Lipoxygenases and Their Role in Plant Physiology
- Emerging role of 12/15-Lipoxygenase in human pathologies
- Absence of Glutathione Peroxidase 4 Affects Tumor
12/15‐lipoxygenase (12‐15LO) is a lipid‐peroxidizing enzyme widely expressed in the central nervous system where it has been involved in the neurobiology of Alzheimer’s disease (AD) because it modulates amyloid beta (Aβ) and APP processing. However, its biological effect on tau protein is unknown. We investigated the effect of 12‐15LO on tau levels and metabolism Several enzymes play a key role in plant growth and development. Among these, lipoxygenases family of enzymes are important. Lipoxygenases are a ubiquitous family of non-heme iron enzymes widely distributed in plants, initiate hydroperoxidation of polyunsaturated fatty acids containing cis, cis-1,4-pentadiene moieties, and produce phytooxylipins. Oxylipins
Inducible GPx4 inactivation in mice and cells revealed 12/15-lipoxygenase-derived lipid peroxidation as specific downstream event, triggering apoptosis-inducing Programmed cell death (PCD) is a basic process of life that is closely related to the growth, development, aging and disease of organisms and is one of the hotspots of life science research today. Conclusion Our data show that inhibition of 12/15-lipoxygenase causes significant cell death reduction after hepatic ischemia and reperfusion by enhancing glutathione metabolism.
Role of ALOX5 protein phosphorylation in cell death. ALOX5 protein phosphorylation plays a central role in ALOX5, and the latter is widely studied in cell death. ALOX5 can affect cell death in two ways. On the one hand, cell death is the central feature of inflammation. The LTs, produced by ALOX5, are a group of proinflammatory mediators. On the other hand, ALOX5 itself is also
Hyperglycaemia increases the activity of 12/15-LO enzyme with a subsequent increase of its bioactive lipid products, 12- and 15-HETE, in retinal endothelial cells. 12/15-LO lipid metabolites increase endothelial intracellular calcium levels that may activate ER
In the humans and mice, six LOX isoforms have been known. 15-LOX, a prototypical enzyme originally found in reticulocytes shares the similarity of amino acid sequence as well as the biochemical property to plant LOX enzymes. 15-LOX-2, which is expressed in epithelial cells and leukocytes, has different substrate specificity in the humans and Abstract Membrane redox state is governed by the complex interplay between oxidation of unsaturated fatty acids and lipophilic antioxidants. In this issue of Cell Metabolism, Seiler et al. (2008) show that a lipid oxidase, 12/15-lipoxygenase, and a membrane antioxidant enzyme, glutathione peroxidase-4, interact to regulate a novel redox-dependent cell death pathway. 12-lipoxygenase (12-LOX) is one of several enzyme isoforms responsible for the metabolism of arachidonic acid and other poly-unsaturated fatty acids to both pro- and anti-inflammatory lipid mediators. Mounting evidence has shown that 12-LOX plays a critical role in the modulation of inflammation at multiple checkpoints during diabetes development.
12/15-lipoxygenase (12/15-LOX) is an enzyme, which oxidizes polyunsaturated fatty acids, particularly omega-6 and -3 fatty acids, to generate a number of bioactive lipid metabolites. A large
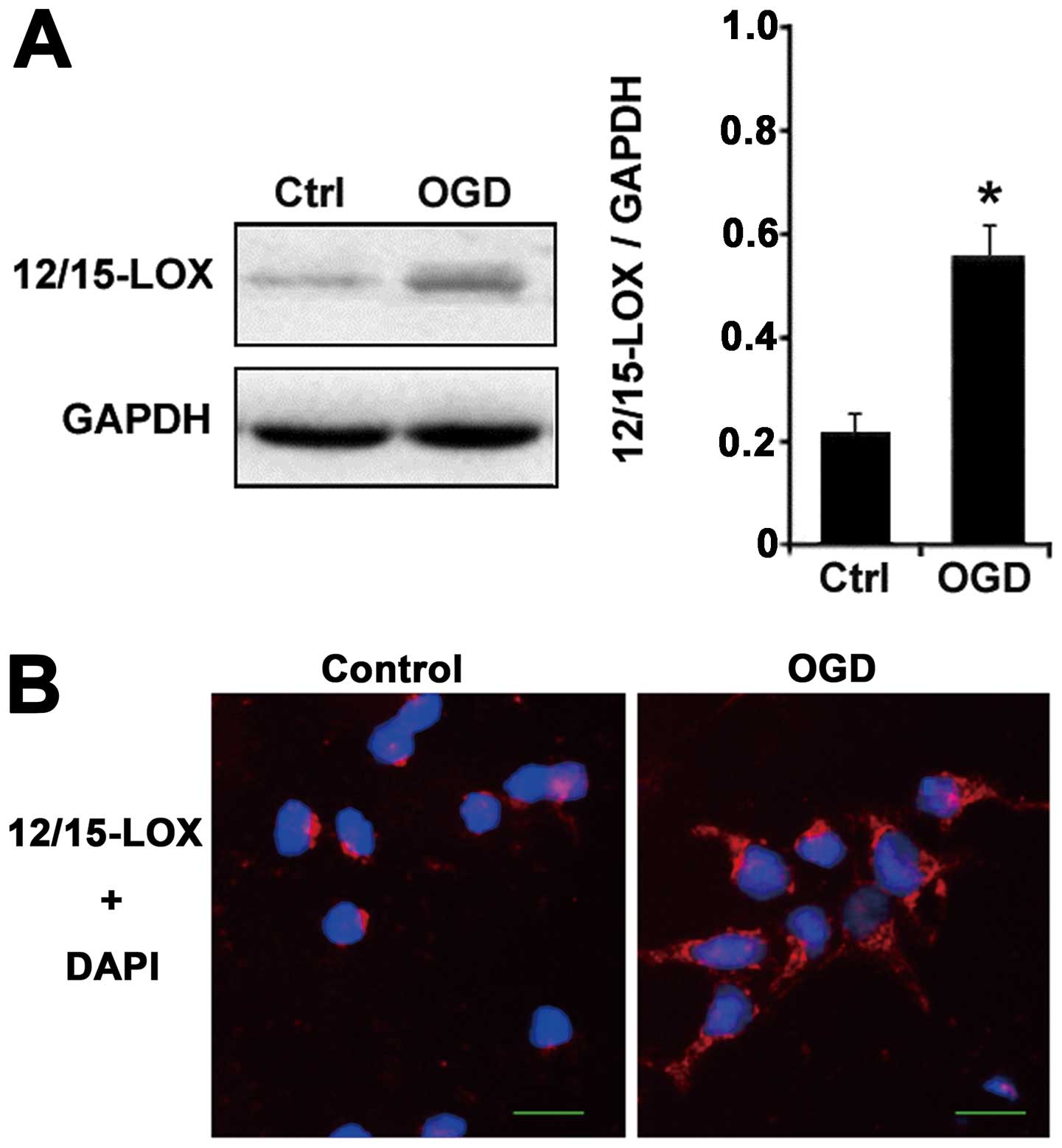
Abstract Background Reactive oxygen species- (ROS-) mediated ischemia-reperfusion injury (IRI) detrimentally impacts liver transplantation and resection. 12/15-Lipoxygenase (12/15-LOX), an antagonistic protein of the glutathione peroxidase 4 (GPX4) signaling cascade, was proven to mediate cell death in postischemic cerebral and myocardial tissue. The aim of this study was to Emerging cellular functions of the lipid metabolizing enzyme 15‐Lipoxygenase‐1 Melis Çolakoğlu 1, Sinem Tunçer 1,2, Sreeparna Banerjee
Arachidonic acid (AA), an ω-6 polyunsaturated fatty acid (PUFA), is predominantly esterified in mammalian cell membrane phospholipids and plays a crucial role in maintaining membrane fluidity and cellular function. 13, 14 Upon cellular stimulation, AA is released via phospholipase-mediated hydrolysis and metabolized through 3 key enzymatic pathways: cyclooxygenases Conclusion Our data show that inhibition of 12/15-lipoxygenase causes significant cell death reduction after hepatic ischemia and reperfusion by enhancing glutathione metabolism.
1. Introduction Lipoxygenases (LOXs) are a well-known class of non-heme, iron containing oxidative enzymes which insert molecular oxygen into long-chain unsaturated fatty acids and derivatives thereof having a cis – cis-1,4-pentadiene motif. There are several types of LOXs in humans including 5-LOX, 12/15-LOX (15-LOX-1), and 15-LOX-2. The 12/15-lipoxygenase enzymes react with fatty acids producing active lipid metabolites that are involved in a number of significant disease states. The latter include type 1 and type 2 diabetes (and associated complications), cardiovascular
Ferroptosis is a form of programmed cell death that is pathogenic to several acute and chronic diseases and executed via oxygenation of polyunsaturated phosphatidylethanolamines (PE) by 15-lipoxygenases (15-LO) that normally use free polyunsaturated fatty acids as substrates. Mechanisms of the altered 15-LO substrate specificity are enigmatic. Abstract Background. Reactive oxygen species- (ROS-) mediated ischemia-reperfusion injury (IRI) detrimentally impacts liver transplantation and resection. 12/15-Lipoxygenase (12/15-LOX), an antagonistic protein of the glutathione peroxidase 4 (GPX4) signaling cascade, was proven to mediate cell death in postischemic cerebral and
The possible involvement of 15- lipoxygenase and its metabolites in the inflammatory process, cell proliferation and death, and immune response has been postulated. Introduction Cancer still ranks the second leading cause of death worldwide despite the emergence of a variety of novel therapeutic options over the past decade. Malignancy of cells reflects an up-regulation of various oncogenic signal cascades that elevate tumor cell proliferation, suppress apoptosis, trigger angiogenesis, and 12/15-Lipoxygenase (12/15-LO) is an enzyme that converts polyunsaturated fatty acids into bioactive lipid derivatives. In this study, we showed that inhibition of 12/15-LO by baicalein (BA
- 16 Of The Best School Websites Built With Wordpress
- 15 Oil And Gas Industry Jobs To Consider
- 15 Gráficos Que Explican Cómo Estamos Acabando Con La Malaria
- 1473: Berlin Hat Höchste Corona-Inzidenz Bundesweit
- 14667 Rav4 52 At Web _ Lehr- und Lernmaterial für die Volksschule
- 1461 Virginia Leder Schuhe In Cherry Red
- 15 Things You Can Legally Do At 16
- 15% Off Sally Beauty Coupons : 15% Off Sally Beauty Coupon, Promo Code
- 14Th Five-Year Plan: Modern Energy System Planning
- 1500Hp Brabham F1 Bmw Turbo! , BMW Brabham BT52 Formula One 1983
- 15 Plumping Skincare Products For A Smooth Complexion
- 159€ Billigflüge Von Frankfurt Am Main Nach Edinburgh 2024
- 15% Nutella Gutschein – 15% Vistaprint Gutschein für Polohemden + 5 weitere Codes
- 15 Compelling Reasons Why You Should Learn A Foreign Language